Maximum quantity allowed is 999
Please select the quantity
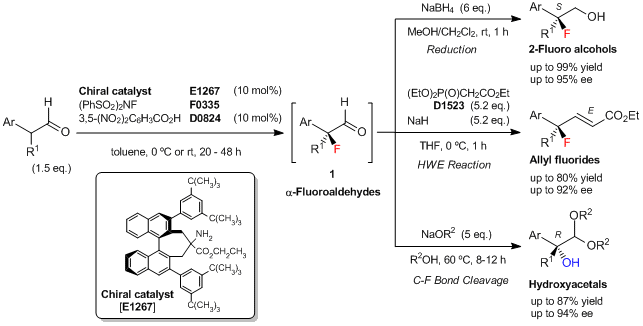
An Enantioselective Fluorination of α-Branched Aldehydes Using Newly Developed Chiral Amine Catalyst
Shibatomi et al. have reported an enantioselective fluorination of α-branched aldehydes using a newly developed chiral binaphthyl-based primary amine catalyst. The asymmetric fluorination of α-branched aldehydes is carried out with N-fluorobenzenesulfonimide (NFSI) in the presence of 10 mol% of the chiral amine catalyst and 10 mol% of 3,5-dinitrobenzoic acid as a co-catalyst to yield the corresponding α-fluoroaldehydes (1). The resulting 1 is difficult to purify, but 1 can be converted into various chiral tertiary fluorides, such as 2-fluoro primary alcohols by the reduction and allyl fluorides by the Horner–Wadsworth–Emmons (HWE) reaction. Additionally, 1 can be converted into the corresponding chiral α-hydroxyacetals with tertiary alcohol moieties via stereospecific C–F bond cleavage.