Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
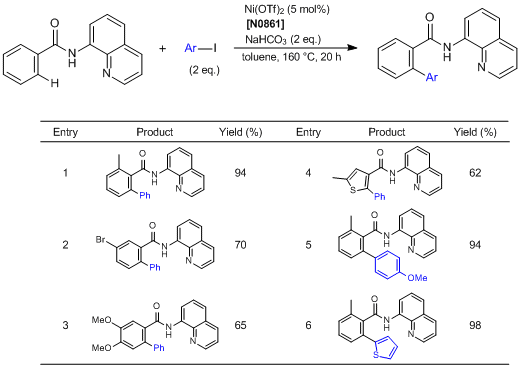
Direct Arylation of C—H Bonds in Aromatic Amides Containing an 8-Aminoquinoline Moiety
Chatani et al. have reported the direct arylation of C—H bonds in aromatic amides containing an 8-aminoquinoline moiety as a directing group. According to their results, the aromatic amides are regioselectively arylated via the cleavage of the ortho C—H bonds by the reaction with aryl iodides using nickel(II) trifluoromethanesulfonate as a catalyst. Various functional group-substituted aromatic amides are tolerated in the reaction. In the reaction using meta-substituted aromatic amides, the arylation proceeds in a highly selective manner at the less hindered C—H bonds, irrespective of the electronic nature of the substituents.