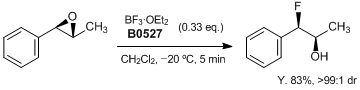Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Chemistry News November 2025 | [Product Highlights] Catechol-containing Methacrylate Monomer for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Stereoselective Ring-Opening Hydrofluorination of Aryl Epoxides
Davies et al. have reported a stereoselective ring-opening hydrofluorination of aryl epoxides with 0.33 equiv of BF3•OEt2 at 20 °C to give rapidly the corresponding syn-fluorohydrins (> 99:1 dr). Mechanistic evidence suggested that a stereoselective SN1-type epoxide ring-opening process involving coordination of BF3 to the oxirane oxygen and transferring of fluorine.