Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [TCIPracticalExample] Suzuki-Miyaura Coupling Using Encapsulated... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 1251667-99-8 | Product Number: C3456
(p-Cymene)bis(mesitylcarboxylato)ruthenium(II)
Purity:
Synonyms:
- Ru(MesCO2)2(p-cymene)
- (p-Cymene)bis(2,4,6-trimethylbenzoato)ruthenium(II)
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 500MG |
$180.00
|
1 | 9 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
Specifications
| Appearance | Light yellow to Yellow to Orange powder to crystal |
| Elemental analysis(Carbon) | 61.00 to 67.00 % |
| NMR | confirm to structure |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
Ruthenium Catalyst for the meta-Selective Substitution Reaction
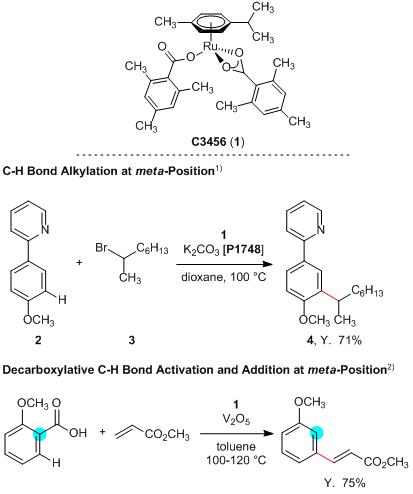
Experimental procedure1):
A mixture of [Ru(MesCO2)2(p-cymene)] (15.0 mg, 0.027 mmol), K2CO3 (1.04 mmol, 2.0 eq.), 2 (96.2 mg, 0.52 mmol) and 3 (282 mg, 1.43 mmol) in dioxane (2 mL) is stirred under N2 for 20 h at 100 °C. EtOAc (50 mL) and H2O (50 mL) are added to the reaction mixture at ambient temperature. The separated aqueous phase is extracted with EtOAc (2 × 50 mL). The combined organic layers are washed with brine (50 mL), dried over Na2SO4 and concentrated in vacuo. The residue is purified by silica gel column chromatography (hexane:EtOAc = 9:1) to yield 4 (108 mg, 71%) as a colorless oil.
A mixture of [Ru(MesCO2)2(p-cymene)] (15.0 mg, 0.027 mmol), K2CO3 (1.04 mmol, 2.0 eq.), 2 (96.2 mg, 0.52 mmol) and 3 (282 mg, 1.43 mmol) in dioxane (2 mL) is stirred under N2 for 20 h at 100 °C. EtOAc (50 mL) and H2O (50 mL) are added to the reaction mixture at ambient temperature. The separated aqueous phase is extracted with EtOAc (2 × 50 mL). The combined organic layers are washed with brine (50 mL), dried over Na2SO4 and concentrated in vacuo. The residue is purified by silica gel column chromatography (hexane:EtOAc = 9:1) to yield 4 (108 mg, 71%) as a colorless oil.
References
- 1)meta-Selective C-H Bond Alkylation with Secondary Alkyl Halides
- 2)Ruthenium(II)-Catalyzed Decarboxylative C-H Activation: Versatile Routes to meta-Alkenylated Arenes
- 3)C(sp3)-H Bond Arylations Catalyzed by Well-Defined [Ru(O2CMes)2(p-cymene)]
PubMed Literature
Product Articles
[Product Highlights] Ruthenium Catalyst for the meta-Selective Substitution ReactionProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




