Maximum quantity allowed is 999
Please select the quantity
CAS RN: 12092-47-6 | Product Number: B1045
Chloro(1,5-cyclooctadiene)rhodium(I) Dimer

Purity:
Synonyms:
- Bis(1,5-cyclooctadiene)dirhodium(I) Dichloride
- 1,5-Cyclooctadiene Rhodium(I) Chloride Dimer
- [Rh(COD)Cl]2
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days |
|---|---|---|---|
| 100MG |
$109.00
|
12 | 21 |
| 1G |
$609.00
|
24 | ≥100 |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | B1045 |
| Molecular Formula / Molecular Weight | C__1__6H__2__4Cl__2Rh__2 = 493.08 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 100MG-Glass Bottle with Plastic Insert (View image), 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 12092-47-6 |
| Reaxys Registry Number | 14860700 |
| PubChem Substance ID | 87563994 |
| MDL Number | MFCD00012415 |
Specifications
| Appearance | Light yellow to Brown powder to crystal |
Properties (reference)
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| H.S.code* | 2843.90-000 |
Application
Rh-catalyzed endo- and Enantioselective Hydroacylation of o-Allylbenzaldehydes
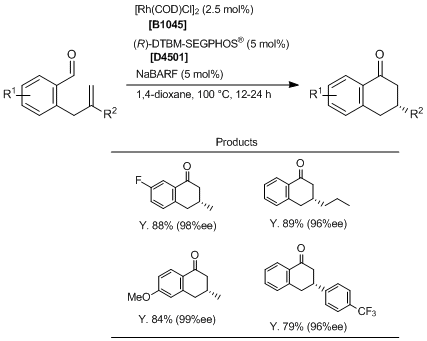
Typical Procedure:
In a nitrogen-filled dry box, the appropriate o-allylbenzaldehyde (0.20 mmol, 1.0 eq.), [Rh(COD)Cl]2 (2.5 mg, 0.0050 mmol, 0.025 eq.), (R)-DTBM-SEGPHOS® (11.8 mg, 0.010 mmol, 0.050 eq.), NaBARF (8.9 mg, 0.010 mmol, 0.050 eq.), and anhydrous 1,4-dioxane (1 mL) are added to a 1-dram vial. The vial is sealed with a PFTE/silicone-lined septum cap and removed from the dry box. The reaction mixture is heated to 100 °C and allowed to stir at this temperature until the reaction is judged to be complete by TLC analysis. The mixture is cooled to room temperature, filtered through a pad of silica gel (eluting with EtOAc), and concentrated under reduced pressure. The crude reaction mixture is purified by flash column silica gel chromatography (hexane/EtOAc or hexane/Et2O) to yield the corresponding products.
In a nitrogen-filled dry box, the appropriate o-allylbenzaldehyde (0.20 mmol, 1.0 eq.), [Rh(COD)Cl]2 (2.5 mg, 0.0050 mmol, 0.025 eq.), (R)-DTBM-SEGPHOS® (11.8 mg, 0.010 mmol, 0.050 eq.), NaBARF (8.9 mg, 0.010 mmol, 0.050 eq.), and anhydrous 1,4-dioxane (1 mL) are added to a 1-dram vial. The vial is sealed with a PFTE/silicone-lined septum cap and removed from the dry box. The reaction mixture is heated to 100 °C and allowed to stir at this temperature until the reaction is judged to be complete by TLC analysis. The mixture is cooled to room temperature, filtered through a pad of silica gel (eluting with EtOAc), and concentrated under reduced pressure. The crude reaction mixture is purified by flash column silica gel chromatography (hexane/EtOAc or hexane/Et2O) to yield the corresponding products.
References
Application
Rhodium-Catalyzed Cross-coupling of Organoboron Compounds with Vinyl Acetate

Typical procedure: Under nitrogen atmosphere, a mixture of an arylboron compounds (0.50 mmol), [RhCl(cod)]2 (6.2 mg, 13 µmol), DPPB (11.7 mg, 28 µmol), and K3PO4 (320 mg, 1.5 mmol) is diluted with toluene (2.0 mL). Alkenyl acetate (1.1 or 2.5 mmol) and tert-amyl alcohol (0.55-1.5 mmol) are added into the resulting suspension at room temperature. The mixture is stirred at 100℃ for 24 h and then diluted with hexane (2.0 mL) or EtOAc (2.0 mL). After the filtration through a Celite pad, solvent is removed from the filtrate under reduced pressure. The residue is purified with a flash column chromatography (EtOAc-hexane) to give the desired product.
References
Application
1,2-Addition of Chiral Secondary and Tertiary Alkyl Trifluoroborate

Typical procedure: A dry Schlenk tube flask is charged with the potassium trifluoroborate salt (0.45 mmol), [RhCl(cod)]2 (2.5 mol%, 3.6 mg) and the aldehyde (0.3 mmol). After cycles of vacuum and nitrogen (3 cycles), deoxygenated 1,4-dioxane (1.4 mL) and deoxygenated H2O (240 µL) are added. The reaction mixture is stirred at 60-100°C and the progress of the reaction is checked by TLC. The mixture is cooled to room temperature, saturated NH4Cl solution is added, and then the mixture is extracted with EtOAc. The combined organic layers are dried over MgSO4. Concentration and purification through silica gel column chromatography gives the products.
References
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Rh-catalyzed endo- and Enantioselective Hydroacylation of o-AllylbenzaldehydesProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



