Maximum quantity allowed is 999
Please select the quantity
Sulfene Equivalent
No.135(July 2008)
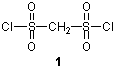

Compound 1 reacts with bases such as Et3N and produces disulfene 2. This compound 2 easily reacts with diene compounds to form [4+2] cycloadduct 3. Therefore, the compound 2 can be used as a trapping reagent for diene compounds because of this property.1) In addition, the compound 2 also reacts with α-olefins to form γ-chloroalkanesulfonyl chloride.2)
References
- 1)[4+2] Cycloaddition reaction of dienes with sulfenes
- 2)Sulfonylation of alkenes
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.