Maximum quantity allowed is 999
Please select the quantity
Stable Isothiocyanating Reagent with Ease of Handling
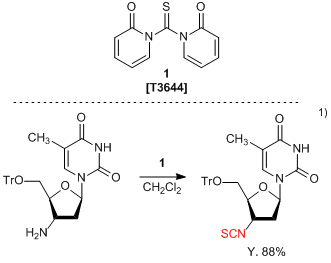
1,1'-Thiocarbonyldi-2(1H)-pyridone (1) is widely used in organosynthetic reactions for the transformation of a primary amino group into an isothiocyanate group.1,2) Both aliphatic and aromatic primary amines can be treated with 1, to produce the corresponding isothiocyanates in high yields. The reaction proceeds under neutral condition and formed byproduct can be removed by aqueous treatment only, allowing for easy workup and purification. Typically, thiophosgene is used to convert an amino group to isothiocyanate, but requires careful handling due to high toxicity and propensity to hydrolyze. In comparison, 1 is an easy-to-handle isothiocyanating reagent with low toxicity and high stability from moisture.
Related Products
References
- 1)Positively Charged Deoxynucleic Methylthioureas: Synthesis and Binding Properties of Pentameric Thymidyl Methylthiourea
- 2)1,1'-Thiocarbonyldi-2,2'-pyridone. A new useful reagent for functional group conversions under essentially neutral conditions