Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
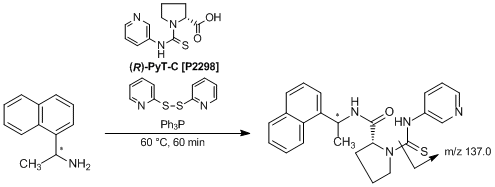
Chiral Derivatization Reagent Possessing a Pyridylthiourea Structure
(R)-PyT-C, which was developed by Toyo’oka et al., is a useful chiral derivatization reagent possessing a pyridylthiourea structure for the enantiomeric separation and the quantitative determination of chiral amines in LC-ESI-MS/MS. In the presence of 2,2'-dipyridyl disulfide and triphenylphosphine as activation reagents, amines are labeled with (R)-PyT-C to afford the corresponding labeled amines. When enantiomeric mixtures of amines are employed, the diastereomeric labeled amines are given. The formed diastereomers are clearly separated by reversed-phase chromatography using an ODS column. Furthermore, the resulting derivatives show a characteristic product-ion (m/z 137.0) derived from (R)-PyT-C, and highly sensitive detection and quantitation are performed from the SRM (selected reaction monitoring) chromatograms. This procedure is also applied to the simultaneous separation and detection of chiral amines. Thus, this analytical procedure is expected to open up a wide range of possibilities for application in the field of metabolomics.