Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Organoboron Reagent Usable for Trifluoromethylating Reactions
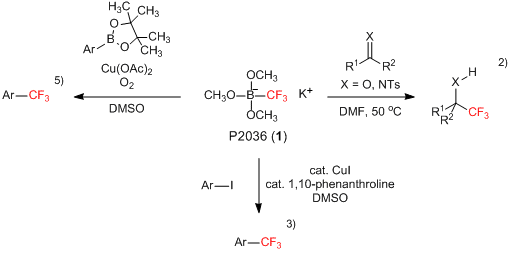
Potassium trimethoxy(trifluoromethyl)borate (1) is a white solid which can be used as a trifluoromethylating reagent with easier handling.1-5) For example, 1 reacts with aldehydes, ketones and imines without α-protons under heating wherein the nucleophilic trifluoromethylation to their carbonyl or imino group proceeds in good yields.2) On the other hand, when substrates having α-protons are treated with 1, the trifluoromethylation competitively proceeds with the self-aldol condensation. Furthermore, by the use of copper salts with 1, cross coupling reactions with aryl iodides3) and aryl pinacolborane derivatives5) successfully proceed and the corresponding trifluoromethyl group-substituted aromatic compounds are given. In this way, 1 is an inexpensive and easier handling nucleophilic trifluoromethylating reagent and is expected to be used in organic synthesis.
Related Products
References
- 1)Perfluoroalkyl borates and boronic esters: new promising partners for Suzuki and Petasis reactions
- 2)Nucleophilic trifluoromethylation with organoboron reagents
- 3)Copper-Catalyzed Trifluoromethylation of Aryl Iodides with Potassium (Trifluoromethyl)trimethoxyborate
- 4)Catalyst-Controlled Regiodivergent C-H Borylation of Multifunctionalized Heteroarenes by Using Iridium Complexes
- 5)Oxidative Trifluoromethylation of Arylboronates with Shelf-Stable Potassium (Trifluoromethyl)trimethoxyborate