Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
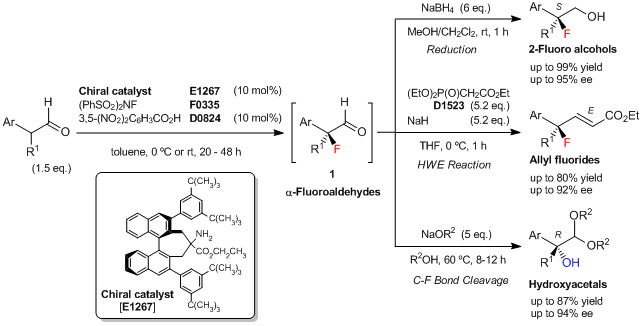
An Enantioselective Fluorination of α-Branched Aldehydes Using Newly Developed Chiral Amine Catalyst
Shibatomi et al. have reported an enantioselective fluorination of α-branched aldehydes using a newly developed chiral binaphthyl-based primary amine catalyst. The asymmetric fluorination of α-branched aldehydes is carried out with N-fluorobenzenesulfonimide (NFSI) in the presence of 10 mol% of the chiral amine catalyst and 10 mol% of 3,5-dinitrobenzoic acid as a co-catalyst to yield the corresponding α-fluoroaldehydes (1). The resulting 1 is difficult to purify, but 1 can be converted into various chiral tertiary fluorides, such as 2-fluoro primary alcohols by the reduction and allyl fluorides by the Horner–Wadsworth–Emmons (HWE) reaction. Additionally, 1 can be converted into the corresponding chiral α-hydroxyacetals with tertiary alcohol moieties via stereospecific C–F bond cleavage.