Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
CAS RN: 72914-19-3 | Product Number: D3134
4,4'-Di-tert-butyl-2,2'-bipyridyl
Purity: >98.0%(GC)
- 4,4'-Di-tert-butyl-2,2'-bipyridine
- dtbpy
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | D3134 |
Purity / Analysis Method 
|
>98.0%(GC) |
| Molecular Formula / Molecular Weight | C__1__8H__2__4N__2 = 268.40 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 72914-19-3 |
| Reaxys Registry Number | 792133 |
| PubChem Substance ID | 172089138 |
| MDL Number | MFCD01863731 |
| Appearance | White to Almost white powder to crystal |
| Purity(GC) | min. 98.0 % |
| Melting point | 159.0 to 163.0 °C |
| Melting Point | 161 °C |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| H.S.code* | 2933.39-000 |
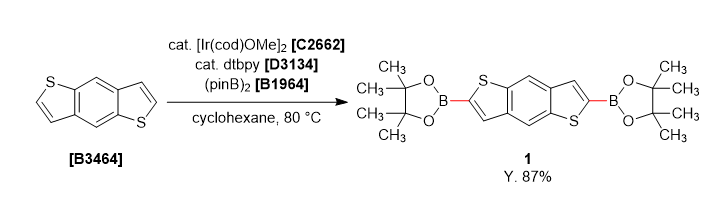
-
Used Chemicals
-
Procedure
-
A solution of dtbpy (27 mg, 0.05 mmol), bis(pinacolato)diboron (1.0 g, 4.0 mmol) and [Ir(cod)OMe]2 (33 mg, 0.025 mmol) in cyclohexane (40 mL) was stirred under nitrogen at room temperature for 10 min. Benzo[1,2-b:4,5-b']dithiophene (1.0 g, 4.0 mmol) was added the mixture and stirred 80 ˚C for 18 hours. The reaction mixture was quenched with water and separated both layers, extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the crude was washed with methanol (20 mL) to give 1 as a white solid (0.771 g, 87% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NMR.
Cyclohexane was bubbled with nitrogen before use.
-
Analytical Data
-
Compound 1
1H NMR (270 MHz, CDCl3); δ 8.36 (s, 2H), 7.90 (s, 2H), 1.39 (s, 24H).
-
Lead Reference
-
- Synthesis and Transistor Application of Bis[1]benzothieno[6,7‑d:6′,7′‑d′]benzo[1,2‑b:4,5‑b′]dithiophenes
References
- 1)Redox-active Esters in Fe-catalyzed C?C Coupling
- 2)Practical Ni-catalyzed Aryl?alkyl Cross-coupling of Secondary Redox-active Esters
- 3)A General Alkyl-alkyl Cross-coupling Enabled by Redox-active Esters and Alkylzinc Reagents

References
Documents
[Research Articles] ortho-Allylation of 1-Arylpyrazoles via Iron-Catalyzed C-H Bond Activation
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts

The requested analytical chart is not available. Sorry for the inconvenience.






