Published TCIMAIL newest issue No.200 | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2025 | [Product Highlights] Cell Culture-Ready Antibiotic... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 6066-82-6 | Product Number: B0249
N-Hydroxysuccinimide [Coupling Reagent for Peptide]
Purity: >98.0%(GC)(T)
Synonyms:
- HOSu
- NHS
Product Documents:
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | B0249 |
Purity / Analysis Method 
|
>98.0%(GC)(T) |
| Molecular Formula / Molecular Weight | C__4H__5NO__3 = 115.09 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic |
| CAS RN | 6066-82-6 |
| Reaxys Registry Number | 113913 |
| PubChem Substance ID | 87563364 |
| SDBS (AIST Spectral DB) | 5311 |
| MDL Number | MFCD00005516 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(GC) | min. 98.0 % |
| Purity(Neutralization titration) | min. 98.0 % |
| Melting point | 98.0 to 102.0 °C |
| Solubility in Dioxane | lmost transparency |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 100 °C |
| Solubility in water | Soluble |
| Solubility (soluble in) | Dioxane |
GHS
Related Laws:
Transport Information:
| H.S.code* | 2928.00-000 |
Application
An Additive for Condensation Reactions
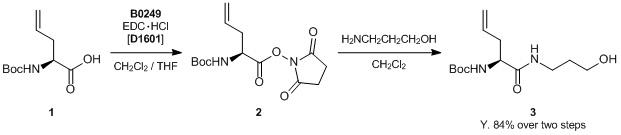
Experimental procedure: A mixture of 1 (3.8 g, 17.5 mmol) and N-hydroxysuccinimide (3.0 g, 26.2 mmol) in CH2Cl2 (100 mL) and THF (50 mL) at 0 °C is treated with EDC・HCl (5.0 g, 26.2 mmol), and the mixture is stirred at the same temperature for 12 h. The reaction is quenched with H2O, and the mixture is partitioned between AcOEt and H2O. The organic phase is washed with saturated aqueous NaCl, dried (Na2SO4), filtered, and concentrated in vacuo. The residue (2) in CH2Cl2 (100 mL) is treated with 3-amino-1-propanol (2.0 mL, 26.2 mmol) at 0 °C and stirred at room temperature for 8 h. The resulting mixture is partitioned between AcOEt and H2O. The organic phase is washed with H2O (twice), saturated aqueous NaCl, dried (Na2SO4), filtered, and concentrated in vacuo. The residue is purified by silica gel column chromatography (40% AcOEt-hexane) to afford 3 as a colorless syrup (4.0 g, 84% over two steps).
References
- Mechanistic Analysis of Muraymycin Analogues: A Guide to the Design of MraY Inhibitors
PubMed Literature
Product Articles
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts

Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.
![N-Hydroxysuccinimide [Coupling Reagent for Peptide] Thumbnail of N-Hydroxysuccinimide [Coupling Reagent for Peptide]](/medias/B0249.jpg?context=bWFzdGVyfHJvb3R8MTUwNzB8aW1hZ2UvanBlZ3xhR1EzTDJoa015ODRPVE16TWpJNE56UXdOak00TDBJd01qUTVMbXB3Wnd8ZjYyZjI3M2NkMDdhYzIxNmNiYTAwNjNiZmI2MjgyYjQzZjNhNGViOTE5NWFhZGM2ODVkOWFhMzllMDgyMGJmMw)
![N-Hydroxysuccinimide [Coupling Reagent for Peptide] Chemical Structure of N-Hydroxysuccinimide [Coupling Reagent for Peptide]](/medias/B0249.jpg?context=bWFzdGVyfHJvb3R8NDU1OTh8aW1hZ2UvanBlZ3xhR1poTDJneE1pODRPVEU1TnpVek5Ea3dORFl5TDBJd01qUTVMbXB3Wnd8YmIzODhjODMwOWFlOGUyNmQwMWVlYmNlNGJmODFmNzgwOTI1YzYxY2I2YWE1YjJmY2YwZWViMTQwNmZiNjViZg)
![N-Hydroxysuccinimide [Coupling Reagent for Peptide] N-Hydroxysuccinimide [Coupling Reagent for Peptide]](/_ui/responsive/theme-tci/images/missing_product_zoom.webp)



