Maximum quantity allowed is 999
CAS RN: 2564-83-2 | Product Number: T1560
2,2,6,6-Tetramethylpiperidine 1-Oxyl Free Radical

Purity: >98.0%(GC)(T)
- TEMPO Free Radical
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 5G |
$60.00
|
≥40 | ≥100 | Contact Us | |
| 25G |
$180.00
|
6 | ≥100 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | T1560 |
| Purity / Analysis Method | >98.0%(GC)(T) |
| Molecular Formula / Molecular Weight | C__9H__1__8NO = 156.25 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Heat Sensitive |
| CAS RN | 2564-83-2 |
| Reaxys Registry Number | 1422418 |
| PubChem Substance ID | 87577318 |
| Merck Index (14) | 9140 |
| MDL Number | MFCD00009599 |
| Appearance | Orange to Brown powder to crystal |
| Purity(GC) | min. 98.0 % |
| Purity(Iodometric Titration) | min. 98.0 % |
| Solubility in Methanol | almost transparency |
| Melting Point | 39 °C |
| Flash point | 67 °C |
| Solubility (soluble in) | Methanol |
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H314 : Causes severe skin burns and eye damage. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dusts or mists. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
| RTECS# | TN8991900 |
| UN Number | UN3263 |
| Class | 8 |
| Packing Group | II |
| H.S.code* | 2933.39-000 |

-
Used Chemicals
-
Procedure
-
To a solution of 1-naphthalenemethanol (306 mg, 2.0 mmol) in dichloromethane (2 mL, 1.0 mol/L) was added TEMPO (31.2 mg, 0.20 mmol), PhI(OAc)2 (709mg, 2.2 mmol) and the mixture was stirred at room temperature for 4 hours. Dichloromethane (12.5 mL), saturated aqueous sodium thiosulfate solution (12.5 mL) was added and the mixture was stirred for 30 minutes. The organic layer was washed with saturated aqueous sodium bicarbonate solution (10 mL), brine (10 mL) and the organic layer was dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (ethyl acetate:hexane = 0:100 - 3:97 on silica gel) to give 1-naphthaldehyde as a yellow liquid (291 mg, 93%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.50).
-
Analytical Data
-
1-Naphthaldehyde
1H NMR (400 MHz, CDCl3); δ 10.41 (s, 1H), 9.26 (d, J = 8.9 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 8.01 (d, J = 6.8 Hz, 1H), 7.93 (d, J = 8.9 Hz, 1H), 7.74-7.57 (m, 3H).
-
Lead Reference
-
- 2-(Hydroxyimino)aldehydes: Photo- and Physicochemical Properties of a Versatile Functional Group for Monomer Design
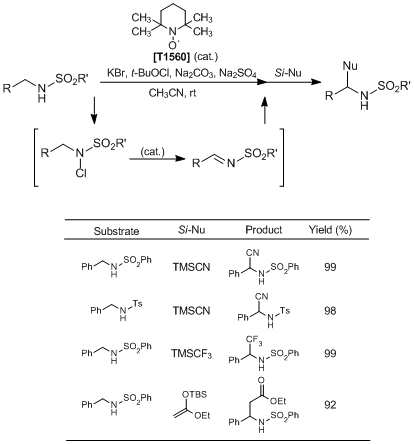
To a solution of N-benzylbenzenesulfonamide (61.8 mg, 0.25 mmol), KBr (14.9 mg, 0.125 mmol), and Na2SO4 (71.0 mg, 0.50 mmol) in MeCN (1.5 mL) is added t-BuOCl (33.9 µL, 0.30 mmol) at room temperature, and the mixture is stirred at room temperature for 1 h. Then, TEMPO (7.8 mg, 0.050 mmol) is added and stirred at room temperature for 30 min. To the solution is added Na2CO3 (26.5 mg, 0.25 mmol), and the mixture is stirred at room temperature for 20 h. Subsequently, TMSCN (93.8 mL, 0.75 mmol) and LiClO4 (2.7 mg, 0.025 mmol) are added to the reaction mixture, and further stirred at room temperature for 22 h. Saturated Na2SO3 aqueous solution (10 mL) is added to the mixture, and the product is extracted with AcOEt (15 mL×3). The organic phase is washed with brine and dried over Na2SO4. The organic phase is concentrated under reduced pressure, and the crude product is purified by column chromatography (hexane : AcOEt = 3 : 1) to give the desired product (67.4 mg, Y. 99%).
References
References
- Indirect electrooxidation of alcohols
- Selective oxidation of monosaccharide derivatives
- Alcohol oxidation in an N-oxoammonium salts-NaBrO2 system
- Alcohol oxidation using organic oxoammonium salts
- Alcohol oxidation with oxygen and cupric ion
- Review
Articles/Brochures
[Research Articles] Nitroxyl-radical-catalyzed Oxidative Coupling of Amides with Silylated Nucleophiles
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.




