Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
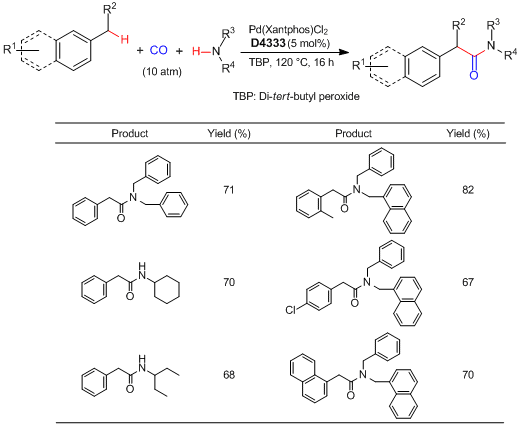
Synthesis of Arylacetamides via Benzylic C-H Bond Activation using a Palladium Catalyst
Huang et al. have reported the palladium-catalyzed oxidative aminocarbonylation via benzylic C-H bond activation. According to their results, under the presence of CO and di-tert-butyl peroxide (=TBP), benzylic C-H bonds of alkyl aromatics are selectively activated by the action of Pd(Xantphos)Cl2 catalyst. Subsequent aminocarbonylation with amines successfully proceeds to afford the corresponding arylacetamides. This reaction can be applied to substrates bearing a halogen substituent, and a subsequent cross-coupling reaction facilitates expedient synthesis of complex arylacetamides. By using this protocol, the marketed drug ibuprofen can be easily obtained.