Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Gelieve het aantal te selecteren
Safe Cyano(nitro)methylating Reagent
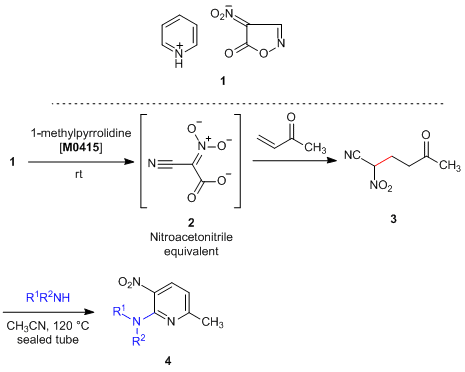
4-Nitro-5(2H)-isoxazolone pyridinium salt (1), which has been developed by Nishiwaki et al., undergoes a ring opening reaction quantitatively under mild conditions upon treatment with a base and is converted to cyano-aci-nitroacetate 2, which works as a synthetic equivalent of nitroacetonitrile (NAN).1) 2 has high solubility in organic solvents whether polar or non-polar. Hence, it is useful as an easy-to-handle cyano(nitro)methylating reagent for synthesis of versatile polyfunctionalized compounds. For example, bifunctional pyridine 4, which possesses vicinally substituted nitro group and amino group, is formed when the Michael adduct 3, which is obtained by adding a vinyl ketone to generated 2, reacts with amines.2) Furthermore, the syntheses of polyfunctionalized heterocyclic compounds such as isoxazolines, isoxazoles, dihydropyridines, and naphthyridines by using 2 have been reported, 2-4) so that 1 is expected to be applied as a safe synthetic equivalent of NAN in the future.
Related Products
References
- 1)Ring opening reaction of the pyridinium salt of 4-nitro-3-isoxazolin-5-one: A preparation of trifunctionalized methane derivatives
- 2)Safe cyano(nitro)methylating reagent – Michael addition of cyano-aci-nitroacetate leading to δ-functionalized α-nitronitriles
- 3)One-pot synthesis of polyfunctionalized isoxazol(in)es
- 4)Synthesis of vicinally functionalized 1,4-dihydropyridines and diazabicycles via a pseudo-intramolecular process
- 5)Review: Nitroisoxazolones Showing Diverse Chemical Behavior: A Use ful Building Block for Polyfunctionalized Systems

