Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Gelieve het aantal te selecteren
An Efficient Chiral Organocatalyst for the Cross-aldol Reaction of Aliphatic Ketones with Aldehydes
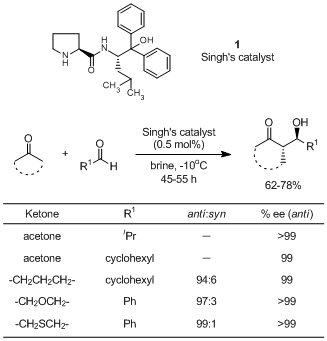
A proline derived chiral organocatalyst, 1, has been developed by Singh and used for chiral cross-aldol reactions in brine. 1-catalyzed direct cross-aldol reactions are widely applicable to various substances such as aliphatic ketones and α-branched aliphatic aldehydes and afford the desired cross-aldol adducts with high enantioselectivities though it is commonly considered to be difficult to use them for direct cross-aldol reactions. Furthermore, using DMF as a solvent, direct cross-aldol reactions of isobutyraldehyde with linear aldehydes successfully proceed to give the cross-aldol adducts enantioselectively. It is assumed that 1 activates the formyl group by hydrogen bonding with both the NH and OH of the amino alcohol moiety while the two phenyl groups of the catalyst 1 induce the stereoselectivity.
References
- Highly Efficient Small Organic Molecules for Enantioselective Direct Aldol Reaction in Organic and Aqueous Media
- Highly Enantioselective Organocatalytic Direct Aldol Reaction in an Aqueous Medium
- Highly Enantioselective Direct Aldol Reaction Catalyzed by Organic Molecules
- Enantioselective Aldol Reactions of Aliphatic Aldehydes with Singh’s Catalyst

