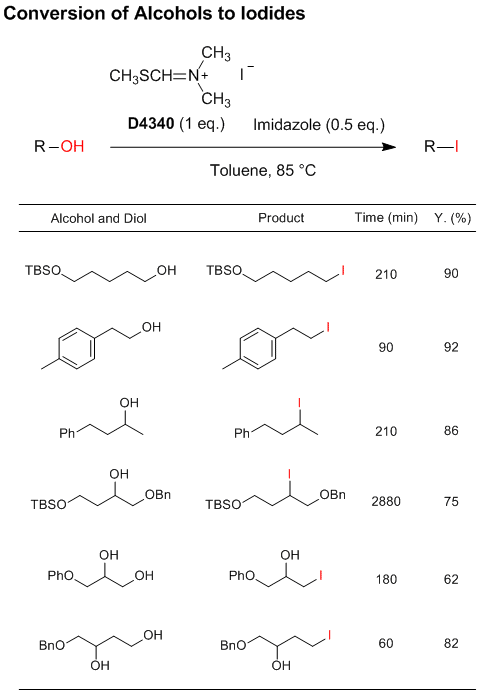
N,N-Dimethyl-N-(methylsulfanylmethylene)ammonium
iodide developed by Porter
et al. is a novel selective iodinating reagent, which can convert primary
and secondary alcohols to the corresponding iodo compounds. In particular, primary alcohols react more rapidly
than secondary alcohols, which allows selective iodination of primary hydroxy groups or in primary-secondary
diols.