Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
| Afmeting | Eenheidsprijs | België | Japan* | Hoeveelheid |
Verzendinformatie

|
|---|---|---|---|---|---|
| 200MG |
€47.00
|
1 | 9 |
|
|
| 1G |
€165.00
|
Neem contact met ons op | ≥40 |
|
*Voorraad beschikbaar in België: Verzending dezelfde dag
*Voorraad beschikbaar in Japan: Raadpleeg de verzendsimulatie voor geschatte levertijden. (met uitzondering van gereguleerde producten en zendingen met droogijs)
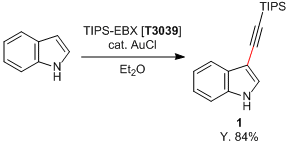
| Artikel # | T3039 |
Zuiverheid / Analysemethode 
|
>98.0%(HPLC) |
| Moleculaire formule / molecuulgewicht | C__1__8H__2__5IO__2Si = 428.39 |
| Fysieke toestand (20 graden C) | Solid |
Opslag condities 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Opslaan onder inert gas | Store under inert gas |
| Te vermijden condities | Light Sensitive,Hygroscopic |
Verpakking 
|
200MG-Glass Bottle with Plastic Insert (Bekijk afbeelding) |
| CAS RN | 181934-30-5 |
| Reaxys registratienummer | 7712699 |
| PubChem product ID | 253660203 |
| MDL-nummer | MFCD18632570 |
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Smeltpunt | 170 °C(dec.) |
| Pictogram |

|
| Signaalwoord | Waarschuwing |
| Gevarenaanduidingen | H315 : Veroorzaakt huidirritatie. H319 : Veroorzaakt ernstige oogirritatie. H413 : Kan langdurige schadelijke gevolgen voor in het water levende organismen hebben. |
| Voorzorgsmaatregelen | P501 : Inhoud/ verpakking afvoeren naar een erkend afvalverwerkingsbedrijf. P273 : Voorkom lozing in het milieu. P264 : Na het werken met dit product de huid grondig wassen. P280 : Beschermende handschoenen/ oogbescherming/ gelaatsbescherming dragen. P337 + P313 : Bij aanhoudende oogirritatie: een arts raadplegen. P332 + P313 : Bij huidirritatie: een arts raadplegen. |
| HS-NR (invoer / uitvoer) (TCI-E) | 2934999090 |

Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.

De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.