Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Gelieve het aantal te selecteren
CAS RN: 917-23-7 | Producten #: T1359
Tetraphenylporphyrin (Chlorin free)

Zuiverheid: >98.0%(HPLC)
Synoniemen
- Tetraphenylporphine (Chlorin free)
- TPP (Chlorin free)
Productdocumenten:
| Afmeting | Eenheidsprijs | België | Japan* | Hoeveelheid |
|---|---|---|---|---|
| 1G |
€139.00
|
12 | ≥60 |
|
*Stock beschikbaar uit voorraad in België leverbaar in 1 tot 3 dagen
*stock beschikbaar uit voorraad in Japan leverbaar in 1 tot 2 weken (met uitzondering van gereguleerde producten en zendingen met droog ijs)
| Artikel # | T1359 |
| Zuiverheid / Analysemethode | >98.0%(HPLC) |
| Moleculaire formule / molecuulgewicht | C__4__4H__3__0N__4 = 614.75 |
| Fysieke toestand (20 graden C) | Solid |
| Opslag condities | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Opslaan onder inert gas | Store under inert gas |
| Te vermijden condities | Air Sensitive |
| CAS RN | 917-23-7 |
| Reaxys registratienummer | 379542 |
| PubChem product ID | 87577126 |
| MDL-nummer | MFCD00011680 |
Specificatie
| Appearance | Orange to Brown to Dark purple powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Tetraphenylchlorin | max. 0.5 % |
| NMR | confirm to structure |
eigenschappen
GHS
Gerelateerde wetten:
| EC-nummers | 213-025-9 |
Transport informatie:
| HS-NR (invoer / uitvoer) (TCI-E) | 2933998090 |
Toepassing
Molecular-weight Controlled Polymerization Using Aluminum-porphyrins
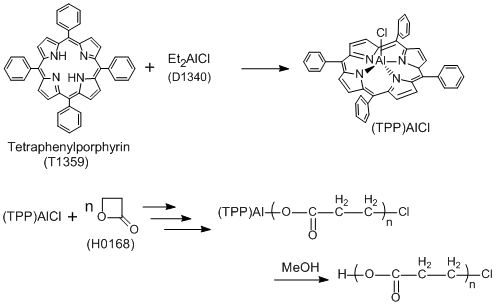
Typical Procedure:
The flask containing tetraphenylporphine ((TPP)H2)(1.0 mmol) is purged with dry N2, and then CH2Cl2 (20 mL) is introduced to dissolve (TPP)H2. To this solution is added Et2AlCl (1.2 mmol, 20% excess to (TPP)H2) by syringe under a N2 atmosphere at room temperature with stirring. After a definite time, the volatile materials are removed from this reaction mixture under reduced pressure to give a purple solid (TPP)AlCl) with metallic luster. CH2Cl2 (20 mL) is introduced to this flask to dissolve the product, to obtain the catalyst solution.
To the catalyst solution ((TPP)AlCl 20.4 mM) with stirring is added β-propiolactone (6.12 mol) by syringe at room temperature in a N2 atmosphere. After a definite time, a large excess of methanol is added to the reaction mixture to stop the polymerization, and then volatile materials are removed under reduced pressure to leave the product. The polymer is isolated as a slightly yellow-green powder by precipitation of the product from chloroform/methanol after shaking the chloroform solution with dilute hydrochloric acid.
The gel permeation chromatogram (GPC) of the resulting polymer shows the weight average molecular weight to the number average molecular weight (Mw/Mn) : 1.26 and the number average molecular weight (Mn) : 3500.
The flask containing tetraphenylporphine ((TPP)H2)(1.0 mmol) is purged with dry N2, and then CH2Cl2 (20 mL) is introduced to dissolve (TPP)H2. To this solution is added Et2AlCl (1.2 mmol, 20% excess to (TPP)H2) by syringe under a N2 atmosphere at room temperature with stirring. After a definite time, the volatile materials are removed from this reaction mixture under reduced pressure to give a purple solid (TPP)AlCl) with metallic luster. CH2Cl2 (20 mL) is introduced to this flask to dissolve the product, to obtain the catalyst solution.
To the catalyst solution ((TPP)AlCl 20.4 mM) with stirring is added β-propiolactone (6.12 mol) by syringe at room temperature in a N2 atmosphere. After a definite time, a large excess of methanol is added to the reaction mixture to stop the polymerization, and then volatile materials are removed under reduced pressure to leave the product. The polymer is isolated as a slightly yellow-green powder by precipitation of the product from chloroform/methanol after shaking the chloroform solution with dilute hydrochloric acid.
The gel permeation chromatogram (GPC) of the resulting polymer shows the weight average molecular weight to the number average molecular weight (Mw/Mn) : 1.26 and the number average molecular weight (Mn) : 3500.
References
- T. Aida, R. Mizuta, Y. Yoshida, S. Inoue, Makromol. Chem. 1981, 182, 1073.

- T. Yasuda, T. Aida, S. Inoue, Macromolecules 1984, 17, 2217.

- S. Asano, T. Aida, S. Inoue, J. Chem. Soc. Chem. Commun. 1985, 1148.

- Y. Watanabe, T. Yasuda, T. Aida, S. Inoue, Macromolecules 1992, 25, 1396.

- M. Komatsu, T Aida, S. Inoue, J. Am. Chem. Soc. 1991, 113, 8492.

- H. Sugimoto, M. Kuroki, T.Watanabe, C. Kawamura, T. Aida, S. Inoue, Macromolecules 1993, 26, 3403.

PubMed Literatuur
Artikelen / Brochures
Productdocumenten (Opmerking: Voor sommige producten zijn geen analytische grafieken beschikbaar.)
Veiligheidsinformatie-blad (VIB)
Selecteer alstublieft taal.
Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Specificatiedocument
Analyse certificaat & andere certificaten
Gelieve het lotnummer in te vullen aub
Er is een onjuist lotummer ingevoerd. Voer alleen 4-5 alfanumerieke tekens vóór het koppelteken in.
Voorbeeldanalysecertificaat
Dit is een voorbeeldanalysecertificaat en vertegenwoordigt mogelijk niet een recent geproduceerde partij van het product.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.
Analytische grafieken
Gelieve het lotnummer in te vullen aub
Er is een onjuist lotummer ingevoerd. Voer alleen 4-5 alfanumerieke tekens vóór het koppelteken in.
De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.






![TPP (=Tetraphenylporphyrin) [Ultra-high sensitive spectrophotometric reagent for Cu] TPP (=Tetraphenylporphyrin) [Ultra-high sensitive spectrophotometric reagent for Cu]](/medias/A5012.jpg?context=bWFzdGVyfHJvb3R8NDUyNTN8aW1hZ2UvanBlZ3xhREJtTDJoaU1DODRPVEk0TlRFeU1UZ3dNalUwTDBFMU1ERXlMbXB3Wnd8MmU4MjU4OThkYTY4ODU5YzFjZmE4YTBjMjQ3NjkwM2UxYTNmNTllN2RkMzMyZDFkODJjOGY3ZTQwZmFhNjk1OA)