Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
| Afmeting | Eenheidsprijs | België | Japan* | Hoeveelheid |
Verzendinformatie

|
|---|---|---|---|---|---|
| 250MG |
€ 79,00
|
1 | Neem contact met ons op |
|
|
| 1G |
€ 214,00
|
Neem contact met ons op | 15 |
|
*Voorraad beschikbaar in België: Verzending dezelfde dag
*Voorraad beschikbaar in Japan: Raadpleeg de verzendsimulatie voor geschatte levertijden. (met uitzondering van gereguleerde producten en zendingen met droogijs)
| Artikel # | B3161 |
Zuiverheid / Analysemethode 
|
>98.0%(T) |
| Moleculaire formule / molecuulgewicht | C__2__4H__5__4P__2Pd = 511.06 |
| Fysieke toestand (20 graden C) | Solid |
Opslag condities 
|
Frozen (<0°C) |
| Opslaan onder inert gas | Store under inert gas |
| Te vermijden condities | Air Sensitive,Heat Sensitive |
Verpakking 
|
1G-Glass Bottle with Plastic Insert (Bekijk afbeelding), 250MG-Glass Bottle with Plastic Insert (Bekijk afbeelding) |
| CAS RN | 53199-31-8 |
| Reaxys registratienummer | 14300595 |
| PubChem product ID | 87560386 |
| MDL-nummer | MFCD03094580 |
| Appearance | White to Yellow to Orange powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| HS-NR (invoer / uitvoer) (TCI-E) | 2843909000 |
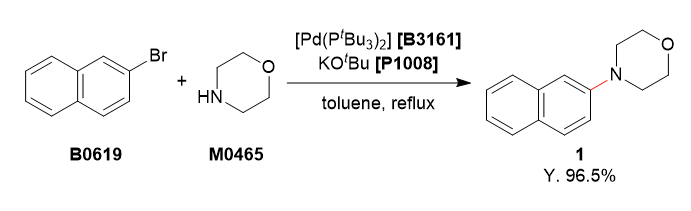
To a 4-necked 200 mL flask was charged with 2-bromonaphthalene (5.20 g, 25.1 mmol, 1.0 equiv.), morpholine (3.27 mL, 37.5 mmol, 1.5 equiv.) and degassed toluene (75 mL). To this solution was added bis(tri-tert-butylphosphine)palladium(0) (256 mg, 0.501 mmol, 2.0 mol%) and potassium tert-butoxide (4.21 g, 37.5 mmol, 1.5 equiv.). The reaction mixture was refluxed for 3 h under argon atmosphere. The reaction mixture was cooled to room temperature and washed with water (50 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (50 mL). The combined organic layers were washed with brine (50 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: hexane/ethyl acetate, 85/15→70/30) to obtain 1 as a white solid (5.17 g, 96.5%).
The reaction mixture was monitored by TLC (hexane/ethyl acetate = 9/1, Rf = 0.70).
1H NMR (400 MHz, CDCl3); δ 7.76–7.69 (m, 3H), 7.42 (t, J = 7.2 Hz, 1H), 7.31 (t, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.13 (s, 1H), 3.92 (t, J = 4.8 Hz, 2H), 3.27 (t, J = 4.8 Hz, 2H).
13C NMR (101 MHz, CDCl3); δ 129.0, 127.6, 127.0, 126.5, 123.7, 119.1, 110.3, 67.1, 50.0.

Typical Procedure: An ampoule is charged with the thiocarbamate (0.442 mmol) and anhydrous degassed toluene (4 mL) is added via gastight syringe under nitrogen with stirring. A J. Youngs valve is fitted and the ampoule is placed into an oil bath pre-equilibrated at 100℃. After ca. 5 minutes the valve is removed and Pd(t-Bu3P)2 (2 mol%, 0.00884 mmol) is added as a solid, the valve is then replaced and the reaction mixture heated for 2.5 hours. A sample of the reaction mixture is removed via gastight syringe and found to contain the desired product (>99%).
Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.

De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.