Graphene, which is one of the nanocarbon materials, consists of all six-membered rings with sp2 carbons having a two-dimensional sheet structure. Graphene has been known for long time, since graphite is formed by combination of graphenes with van der Waals force. However, details of the properties were unclear until late years, because an isolation procedure of graphene from graphite was not well developed for long time. Geim and Novoselov et al. in 2004 successfully isolated a thin-flake graphene by a simple procedure. They used a tape to peel off a graphene layer from highly oriented pyrolytic graphite (HOPG) and then the peeled graphene layer is stuck on a substrate. After this observation,1) studies of graphene have proved the particular characteristics of electronic, mechanical, and chemical properties. Geim and Novoselov won their joint Nobel prizes in physics in 2010 for their contributions.
The most characteristic feature of graphene is its electrical property. The electron mobility in the graphene layer is 100 times greater than that of silicon.2) Accordingly, we can expect to develop a transistor with high-mobility and low-power consumption. Graphene may be promising for a next generation channel material that is useful for LSI (large-scale integration). In addition, the physical strength of graphene is 100 times greater than that of iron. The current density tolerance is much better than that of copper, thus it is expected to be an electrical wire transporting large currents.3)
Electrons in the graphene behave as massless Dirac fermions as similar to neutrinos,4) and demonstrate a quantum Hall effect at room temperature.5) Graphene is an ideal material for spintronics, since there are small spin orbit interactions and the negligible nuclear magnetic moment of carbon. Hybridization of graphene and a ferromagnetic material is developed for the application of an information processor using the electron spin (spintronics device).6)
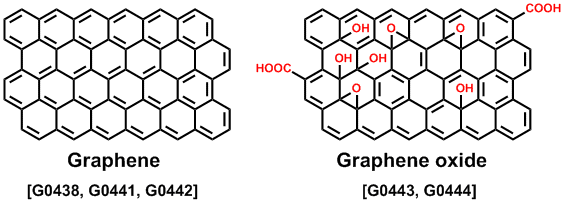
The fabrication procedure for graphene is peeling off the layer from HOPG, a chemical vapor deposition (CVD)7) as well as reduction of a graphene oxide (GO).8) There are various synthetic methods of making GO, and the properties and applications depend on the degree of oxidation. GO disperses in water and several polar solvents, because the structure of GO normally includes hydroxyl, epoxy, and carboxyl groups on the graphene sheet. Accordingly, a GO thin film can be fabricated on a substrate by a solution-process. The reduction of GO provides a reduced graphene oxide (rGO), but it is not a perfect graphene. The rGO contains a few oxygen components and defects on the graphene structure. Although GO is an insulator because there are sp3 carbon atoms, rGO is conductive. Therefore the rGO is expected to be an electrode material. A water dispersion of GO is used as a lubricant to reduce friction on metal surfaces.9) GO-supported metal catalysts were developed for a cross-coupling reaction and hydrogenation.10,11) We can introduce several functional groups on GO because there are oxygen-based groups. These GO derivatives may be useful for luminescent materials and biosensors.12,13)



