Maximum quantity allowed is 999
Please select the quantity
Arylboronic Acid
No.119(March 2004)
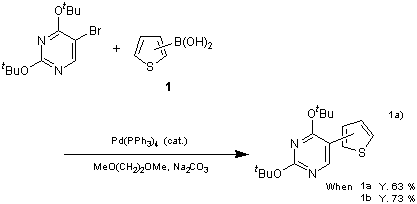
The introduction of a thienyl group often enhances the biological activities as well as their electrical conductivities in certain molecules, and thus a large number of compounds have been experimented. Thiopheneboronic acids 1 are useful reagents for the introduction of this thienyl group to a molecule. As shown in the following examples, the Suzuki-Miyaura coupling reactions of 1 with 5-bromouracil derivatives in the presence of palladium catalyst furnished 5-(2-thienyl) uracil derivatives in a 63% and 73% yield, respectively.1a)
References
- 1)Synthesis of various thienyl compounds
- a)S. Gronowitz, A.-B. Honfeldt, V. Kristjansson, T. Musil, Chemica Scripta. 1986, 26, 305.
- b)M. Allegretti, A. Arcadi, F. Marinelli, L. Nicolini, Synlett 2001, 609.

- c)S. Kotha, A. K. Ghosh, Synlett 2002, 451.

The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.