Maintenance Notice (3:15 AM - 2:55 PM May 24, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Materials Science News May 2025 | [Product Highlights] Endogenous Biotin-Blocking Reagent... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
Diamidite for the Synthesis of Phosphodiesters
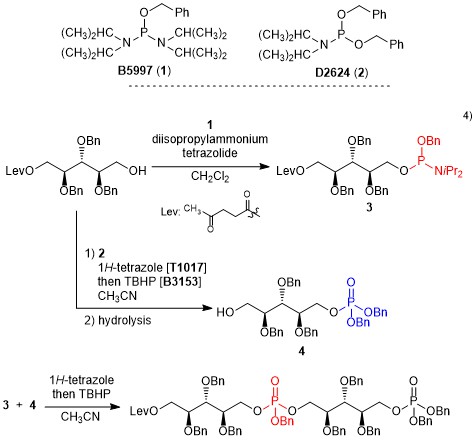
Benzyl N,N,N',N'-tetraisopropylphosphorodiamidite (1) is reportedly used in the synthesis of phosphodiesters1) and phosphorylation of inositols2) and nucleosides.3) 1 promptly reacts with alcohols in the presence of a base like 1H-tetrazole to afford a phosphoramidite. Since phosphoramidite can react with another alcohol, a phosphite trimester with different alcohol species can be synthesized. Successive oxidation of the phosphorus atom and deprotection gives a phosphodiester. Whereas, dibenzyl N,N-diisopropylphosphoramidite (2) reacts with only one alcohol molecule, phosphate monoesters can be synthesized in a same manner. Thus, 1 and 2 are properly used in a synthesis of phosphate esters. For instance, Stehle and Peschel et al. have synthesized the ribitol phosphate dimer by using 1 and 2.4)
Related Products
- B5997
- Benzyl N,N,N',N'-Tetraisopropylphosphorodiamidite
- D2624
- Dibenzyl N,N-Diisopropylphosphoramidite
References
- 1) A Simple and Effective Chemical Phosphorylation Procedure for Biomolecules
- 2) Synthetic Approaches to Heavily Lipidated Phosphoglyceroinositides
- 3) Transition State-Based Sialyltransferase Inhibitors: Mimicking Oxocarbenium Ion by Simple Amide
- 4) Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity