Maintenance Notice (10:30 PM June 7 - 2:00 AM June 8, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News June 2025 | [Product Highlights] Benzoquinone Derivative Useful for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
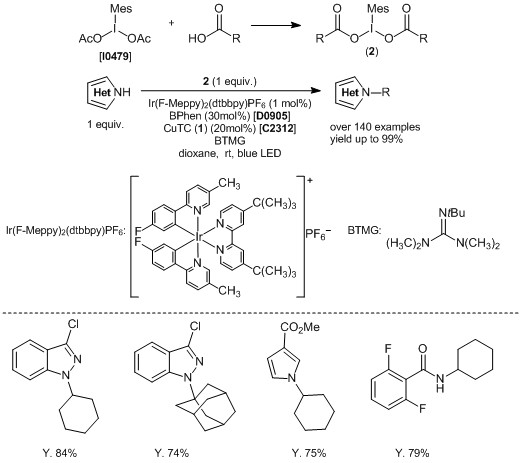
Copper/photoredox-catalyzed Decarboxylative sp3 C–N Coupling Reaction of N-Heteroaromatics
MacMillan and co-workers have recently utilized copper(I) 2-thiophenecarboxylate (1) as a catalyst for a C-N coupling reaction of N-heteroaromatic derivatives in the presence of photoredox catalyst. For example, iodomesitylene dicarboxylate (2) prepared from iodomesitylene diacetate and alkylcarboxylic acids reacts with N-heteroaromatic derivatives. The Ir complex, 1 and bathophenanthroline act as a catalyst to give the corresponding N-alkyl heteroaromatic products in good yields. This reaction can proceed when using a carboxylic acid neighboring a sterically hindered alkyl group such as adamantyl group. Furthermore, the C-N coupling is utilized by the amide nitrogen, carbonate and sulfone amides. In this way, this reaction is expected to be use in late-stage C-N coupling of research of pharmaceuticals, as well as late-stage total synthesis.
Related Products
Reference
- Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis