Maximum quantity allowed is 999
Please select the quantity
CAS RN: 3375-31-3 | Product Number: A1424
Palladium(II) Acetate

Purity: >98.0%(T)
Synonyms:
- Acetic Acid Palladium(II) Salt
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
$116.00
|
≥60 | ≥100 | Contact Us | |
| 5G |
$469.00
|
24 | ≥100 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | A1424 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__4H__6O__4Pd = 224.51 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 3375-31-3 |
| Reaxys Registry Number | 3602276 |
| PubChem Substance ID | 87562878 |
| SDBS (AIST Spectral DB) | 12841 |
| Merck Index (14) | 6991 |
| MDL Number | MFCD00012453 |
Specifications
| Appearance | Orange to Brown powder to crystal |
| Purity(Chelometric Titration) | 98.0 to 102.0 % |
| Elemental analysis(Nitrogen) | max. 3.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 205 °C |
| Maximum Absorption Wavelength | 400 nm (EtOH) |
| Solubility (soluble in) | Acetone |
GHS
| Pictogram |



|
| Signal Word | Danger |
| Hazard Statements | H318 : Causes serious eye damage. H317 : May cause an allergic skin reaction. H410 : Very toxic to aquatic life with long lasting effects. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P261 : Avoid breathing dust. P273 : Avoid release to the environment. P272 : Contaminated work clothing should not be allowed out of the workplace. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P391 : Collect spillage. P362 + P364 : Take off contaminated clothing and wash it before reuse. P333 + P313 : If skin irritation or rash occurs: Get medical advice/ attention. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
Related Laws:
| RTECS# | AJ1900000 |
Transport Information:
| UN Number | UN3077 |
| Class | 9 |
| Packing Group | III |
| H.S.code* | 2843.90-000 |
Application
Regioselective C-H Coupling Reaction of Benzo[h]quinoline and Arene Compounds

Typical Procedure:
Palladium(II) acetate (9.6 mg, 0.048 mmol), 1,4-benzoquinone (23.7 mg, 0.22 mmol), silver(I) carbonate (237.2 mg, 0.86 mmol) and benzo[h]quinoline (0.43 mmol) are weighed into a 20 mL scintillation vial. After adding 1,2-dichlorobenzene (3.75 mL), 125 µL of DMSO (1.45 mmol) is added. The resulting mixture is sealed with a teflon-lined cap, and vigorously stirred at 130 °C. The reaction mixture turns grey/brown upon heating. The reaction is cooled to room temperature, filtered through a plug of silica, and the silica is washed with copious AcOEt (150 mL). The filtrate is concentrated and then evaporated to dryness under high vacuum, and the resulting residue is purified by column chromatography (SiO2, AcOEt : hexane = 2 : 98) to afford the desired product as a pale yellow solid (130 mg, Y. 93%).
Palladium(II) acetate (9.6 mg, 0.048 mmol), 1,4-benzoquinone (23.7 mg, 0.22 mmol), silver(I) carbonate (237.2 mg, 0.86 mmol) and benzo[h]quinoline (0.43 mmol) are weighed into a 20 mL scintillation vial. After adding 1,2-dichlorobenzene (3.75 mL), 125 µL of DMSO (1.45 mmol) is added. The resulting mixture is sealed with a teflon-lined cap, and vigorously stirred at 130 °C. The reaction mixture turns grey/brown upon heating. The reaction is cooled to room temperature, filtered through a plug of silica, and the silica is washed with copious AcOEt (150 mL). The filtrate is concentrated and then evaporated to dryness under high vacuum, and the resulting residue is purified by column chromatography (SiO2, AcOEt : hexane = 2 : 98) to afford the desired product as a pale yellow solid (130 mg, Y. 93%).
References
- Catalytic and highly regioselective cross-coupling of aromatic C–H substrates
Application
Aripiprazole: A Partial Agonist at Dopamine D2 and Serotonin 5-HT1A Receptors

References
Application
Acadesine (AICAR): AMP-Activated Protein Kinase Activator

References
Application
Synthesis of aromatic esters via Pd-catalyzed decarboxylative coupling with bidentate phosphine ligands
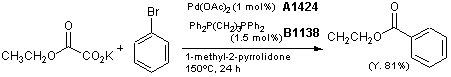
Typical procedure:
A 10 mL oven-dried Schlenk tube is charged with Pd(OAc)2 (0.005 mmol), 1,3-bis(diphenylphosphino)propane (0.0075 mmol), and potassium ethyl oxalate (0.75 mmol). The tube is evacuated and backfilled with argon. Bromobenzene (0.5 mmol) and 1-methyl-2-pyrrolidone (1.0 mL), are added by syringe at room temperature. The tube is then sealed and the mixture is allowed to stir under 1 atm of argon at 150°C for 24 h (the tube is connected to the Schlenk line which is filled with argon so that CO2 can be released to air).
A 10 mL oven-dried Schlenk tube is charged with Pd(OAc)2 (0.005 mmol), 1,3-bis(diphenylphosphino)propane (0.0075 mmol), and potassium ethyl oxalate (0.75 mmol). The tube is evacuated and backfilled with argon. Bromobenzene (0.5 mmol) and 1-methyl-2-pyrrolidone (1.0 mL), are added by syringe at room temperature. The tube is then sealed and the mixture is allowed to stir under 1 atm of argon at 150°C for 24 h (the tube is connected to the Schlenk line which is filled with argon so that CO2 can be released to air).
References
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




