Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
A Water-Soluble Chiral Shift Reagent for Use
in High-Field NMR
No.123(July 2005)
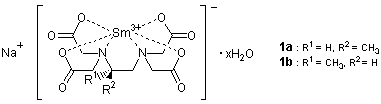
There have been developed various methods of determining the enantiomeric purity and absolute configuration of chiral compounds using NMR, the most common and versatile analytical method in organic chemistry. As one of these, there is the method to resolve enantiomer signals by using paramagnetic chiral lanthanide shift reagents. For example, the europium propylenediaminetetraacetate complex (Eu-pdta) was reported to be useful for assigning the absolute configurations of α-amino acids and α-hydroxy acids in D2O.1) However, it has been well known that lanthanide shift reagents generally have the drawback of causing line broadening, which is more serious when they are used in stronger magnetic field. Eu-pdta often caused heavy line broadening especially for the signals of α-amino acids even with 90 MHz 1H NMR and it could not be used for these substrates in high-field NMR because of serious line broadening. In recent years, Kabuto and co-workers have demonstrated that the Samarium complex (Sm-pdta) 1 is not as likely to cause line broadening in high-field NMR as Eu-pdta does and can be also used in determining absolute configurations of the α-amino acids.2) Observation of the chemical shift nonequivalence for several protons enabled by the use of high-resolution NMR increases the reliability of the assignment as in modified Mosher method.
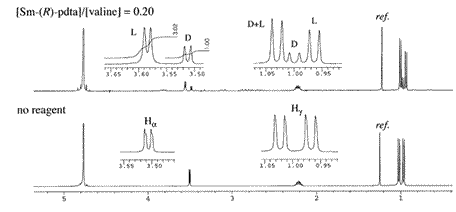

Fig.1. 1H NMR spectra (400 MHz) of valine (0.06 M, [D]/[L] = 1/2.85) in D2O at pH 9.4.
Resolution of the enantiomer signals of α-amino acids
NMR measurement is carried out on D2O solutions of α-amino acids of pH 9-10, near the pKa of the substrates, where the best resolution is possible. The pH of the sample solution is adjusted with D2O solutions of NaOD (~2M and ~0.2 M for fine adjustment, added with a micropipet), and a D2O solution of DCl, if necessary. Use of the buffer solutions containing anions such as phosphate and carbonate cannot be recommended because of their possible coordination to the lanthanide ion. When a sample solution contains both D-isomer and L-isomer, 1 is directly added in small amounts to the sample tube (when the concentration of the amino acid is 0.06 M, an amount of reagent is approximately 5-20 mol% of a substrate), and is dissolved by shaking the tube. Figure 1 shows an example of the NMR resolution of the enantiomer signals of valine utilizing the above procedure (1H NMR: 400 MHz; [valine] = 0.06M; D/L ratio = 1/2.85; pH 9.4; [1a]/[valine] = 0.2). Since the complex 1 itself also possesses several broad signals in the range of 2-4 ppm, it is not the appropriate reagent to use for determining the enantiomeric purity. However, as in the above example, when the enantiomer signals can be resolved at the baseline without overlapping with the signals of the reagent, then the approximate D/L ratio can be obtained from the ratio of the integration (D/L = 1/3.02 in the above case).
NMR measurement is carried out on D2O solutions of α-amino acids of pH 9-10, near the pKa of the substrates, where the best resolution is possible. The pH of the sample solution is adjusted with D2O solutions of NaOD (~2M and ~0.2 M for fine adjustment, added with a micropipet), and a D2O solution of DCl, if necessary. Use of the buffer solutions containing anions such as phosphate and carbonate cannot be recommended because of their possible coordination to the lanthanide ion. When a sample solution contains both D-isomer and L-isomer, 1 is directly added in small amounts to the sample tube (when the concentration of the amino acid is 0.06 M, an amount of reagent is approximately 5-20 mol% of a substrate), and is dissolved by shaking the tube. Figure 1 shows an example of the NMR resolution of the enantiomer signals of valine utilizing the above procedure (1H NMR: 400 MHz; [valine] = 0.06M; D/L ratio = 1/2.85; pH 9.4; [1a]/[valine] = 0.2). Since the complex 1 itself also possesses several broad signals in the range of 2-4 ppm, it is not the appropriate reagent to use for determining the enantiomeric purity. However, as in the above example, when the enantiomer signals can be resolved at the baseline without overlapping with the signals of the reagent, then the approximate D/L ratio can be obtained from the ratio of the integration (D/L = 1/3.02 in the above case).
Determination of absolute configuration:
(A) Enantiomeric mixture: When measurements of different types of α-amino acid (D/L = 1/2) are made under the above conditions, resolution of the enantiomer NMR signals yields the following results (See Table 1). The chemical shift differences between the enantiomer signals were determined for the enantiomeric mixtures of various α-amino acids under the conditions described above. Some results are shown in Table 1.
(A) Enantiomeric mixture: When measurements of different types of α-amino acid (D/L = 1/2) are made under the above conditions, resolution of the enantiomer NMR signals yields the following results (See Table 1). The chemical shift differences between the enantiomer signals were determined for the enantiomeric mixtures of various α-amino acids under the conditions described above. Some results are shown in Table 1.
Table 1. Resolution of enantiomer signals of amino acids in the presence of 1a.

Here ΔΔδ is δ(L)-δ(D), and δ(L) and δ(D) indicate the chemical shifts of 1H signal due to L- and D-amino acids in the presence of 1a, respectively. As shown in Figure 2, in the presence of 1a, the H signals of the D-isomers appeared more upfield than those of L-isomers, while the signals of side chain protons of L-isomer resonated upfield compared with those of their counterparts. This relation was observed for almost all of the amino acids examined. Therefore, by observing the separation of H and side chain proton signals, it is possible to assign the absolute configuration of α-amino acids including those with unknown configuration.
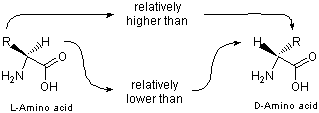
Fig. 2. Relative position of proton signals of amino acid enantiomers in the presence of 1a.
(B) Single enantiomer: Actual samples are often enantiopure. It is possible to determine the absolute configuration of a single enantiomer, by conducting the two separate measurements in the presence of 1a and 1b and comparing chemical shifts of the corresponding signals. This is because the chemical shifts of the signals of the enantiomer at hand in the presence of 1b are the same as those of its enantiomer measured in the presence of 1a. The chemical shifts of the 1H signals of amino acid are sensitive to concentration, temperature and pH, thus strict control of these conditions is required as well as controlling the equivalence of each reagent. The optimal procedures is to first prepare two sample tubes containing equivalent amounts of a pH-adjusted sample solution. To one and the other tubes, add separately the same amount of D2O solutions of 1a and 1b (pH adjusted to 8), whose concentrations are the same, using microsyringes to conduct the measurements.
This method has also been applied to α-hydroxy acids at pH ~ 5 and the relation for the side chain protons shown in Figure 2 was consistently observed.3)
(B) Single enantiomer: Actual samples are often enantiopure. It is possible to determine the absolute configuration of a single enantiomer, by conducting the two separate measurements in the presence of 1a and 1b and comparing chemical shifts of the corresponding signals. This is because the chemical shifts of the signals of the enantiomer at hand in the presence of 1b are the same as those of its enantiomer measured in the presence of 1a. The chemical shifts of the 1H signals of amino acid are sensitive to concentration, temperature and pH, thus strict control of these conditions is required as well as controlling the equivalence of each reagent. The optimal procedures is to first prepare two sample tubes containing equivalent amounts of a pH-adjusted sample solution. To one and the other tubes, add separately the same amount of D2O solutions of 1a and 1b (pH adjusted to 8), whose concentrations are the same, using microsyringes to conduct the measurements.
This method has also been applied to α-hydroxy acids at pH ~ 5 and the relation for the side chain protons shown in Figure 2 was consistently observed.3)
References
- 1)K. Kabuto, Y. Sasaki, J. Chem. Soc., Chem. Commun. 1987, 670.

- 2)A. Inamoto, K. Ogasawara, K. Omata, K. Kabuto, Y. Sasaki, Org. Lett. 2000, 2, 3543.

- Tokyo Kasei Kogyo Co., LTD., JP2002-80437.
- 3)K. Omata, K. Horie, K. Ogasawara, K. Kabuto, Y. Sasaki, Abstract of 8th International conference on Circular Dichroism 2001, p.78.
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

