Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Merci de sélectionner la quantité
CAS RN: 1109-15-5 | Numéro de produit: T2313
Tris(pentafluorophenyl)borane

Pureté: >98.0%(NMR)
Synonymes
Documents de produit:
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 1G |
€93.00
|
19 | ≥100 |
|
| 5G |
€362.00
|
9 | ≥80 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | T2313 |
| Pureté / Méthode d'analyse | >98.0%(NMR) |
| Formule moléculaire / poids moléculaire | C__1__8BF__1__5 = 511.98 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Frozen (<0°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Hygroscopic,Heat Sensitive |
| Emballage Et Conteneur | 1G-Glass Bottle with Plastic Insert (Voir l'image) |
| CAS RN | 1109-15-5 |
| Numéro de registre de Reaxys | 2931347 |
| Identifiant de la substance PubChem | 87558752 |
| Indice Merck (14) | 9755 |
| Numéro MDL | MFCD00269813 |
Spécifications
| Appearance | White to Gray to Brown powder to crystal |
| Purity(NMR) | min. 98.0 atom% |
| Water | max. 1.0 % |
Propriétés
| Point de fusion | 128 °C |
| Longueur d'onde maximale | 306(Toluene) nm |
| Solubilité (soluble dans) | Toluene |
SGH
| Pictogramme |

|
| Mot de signal | Attention |
| Mentions de danger | H315 : Provoque une irritation cutanée. H319 : Provoque une sévère irritation des yeux. |
| Conseils de prudence | P264 : Se laver la peau soigneusement après manipulation. P280 : Porter des gants de protection/ un équipement de protection des yeux/ du visage. P302 + P352 : EN CAS DE CONTACT AVEC LA PEAU: Laver abondamment à l’eau. P337 + P313 : Si l'irritation oculaire persiste: consulter un médecin. P362 + P364 : Enlever les vêtements contaminés et les laver avant réutilisation. P332 + P313 : En cas d'irritation cutanée: consulter un médecin. |
Lois connexes:
Informations de transport:
| N ° SH (import / export) (TCI-E) | 2931900090 |
Application
Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane
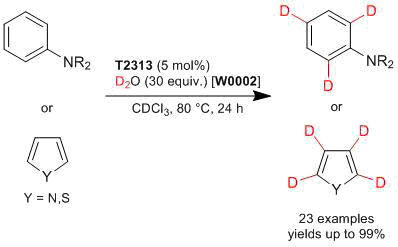
Experimental procedure: N,N-Dibenzylaniline (140 mg, 0.513 mmol), tris(pentafluorophenyl)borane (17.0 mg, 0.033 mmol), D2O (306 mg, 15.3 mmol) are placed in a sealed tube. The reaction mixture is stirred at 80 °C for 24 h. After the reaction is completed, the reaction mixture is purified by silica gel column chromatography (cyclohexane:ethylacetate = 20:1) to afford N,N-dibenzylaniline-2,4,6-d3 (134 mg) in 95% yield.
References
- B(C6F5)3‑Catalyzed Regioselective Deuteration of Electron-Rich Aromatic and Heteroaromatic Compounds
Application
High Throughput Sequence-controlled Oligosiloxane Synthesis

References
- By-Product-Free Siloxane-Bond Formation and Programmed One-Pot Oligosiloxane Synthesis
Application
Frustrated Lewis Pair (FLP)-induced Hydrogenations of Silyl Enol Ethers

References
- Heterolytic dihydrogen activation with the 1,8-bis(diphenylphosphino)naphthalene/B(C6F5)3 pair and its application for metal-free catalytic hydrogenation of silyl enol ether
Application
Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)

Synthesis of multisubstituted silanes:
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
References
- Formal SiH4 chemistry using stable and easy-to-handle surrogates
Application
Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)

Typical procedure (R, R’ = n-Pr): 4-Heptanone (1.00 g, 8.76 mmol) is weighed into a 125 mL reactor. Subsequently, B(C6F5)3 (0.224 g, 0.43 mmol) dissolved in Et2O (14.3 mg, 20 mL, 0.19 mol) is added to the reactor. The reactor is sealed and attached to a hydrogen gas line. The flask is purged ten times at 15 atm with hydrogen gas. The reactor is then pressurized with 60 atm hydrogen gas and placed in an oil bath for 12 h at 70 °C. The reactor is slowly vented and all the volatiles are collected by vacuum distillation while cooling the collected distillate with liquid nitrogen. The solvent is removed by applying a gentle stream of N2 gas to give 4-heptanol (886 mg, 87% yield, and 99 % conversion determined by 1H NMR).
References
- T. Mahdi, D. W. Stephan, J. Am. Chem. Soc. 2014, 136, 15809.

- Highlighted in Chem. Eng. News 2014, 92 (44), 8.

Application
Metal-Free Hydrogenation of N-Phenyl Aromatic Rings

Typical procedure (Entry 1): A 25 mL glass bomb equipped with Teflon screw cap is charged with a solution of B(C6F5)3 (37.9 mg, 1 eq.) and N-isopropylaniline (10.0 mg, 0.074 mmol) in toluene (1 mL). The reaction tube is degassed three times through a freeze-pump-thaw cycle on the vacuum/H2 line and filled with H2 (4 atm) at -196 °C. The reaction bomb is placed in a 110 °C oil bath for 36 h. The toluene is removed under reduced vacuum to yield a white precipitate. The product is washed with pentane (2 x 2 mL) and dried under reduced pressure to give [iPrNH2Cy][HB(C6F5)3] (93 %).
References
- 1)T. Mahdi, Z. M. Heiden, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 4088.

- 2)D. W. Stephan, G. Erker, Angew. Chem., Int. Ed. 2010, 49, 46.

Application
Metal-free Hydrogenation of Imines Catalyzed by B(C6F5)3

Typical Procedure: In a glovebox, B(C6F5)3 (18.2 mg, 0.0355 mmol, 20 mol%) is dissolved in dry diisopropylamine (2.5 mL, 1.8 g, 17 mmol) and the solution is added to N-benzylidene-tert-butylamine (28.7 mg, 0.177 mmol, 1 eq.). The resulting solution is transferred to a 25 mL bomb with a sealable Teflon tape and magnetic stirbar. The reaction vessel is sealed, removed from the glovebox and stirred at 100°C for 24 h after which it is cooled to room temperature. The reaction mixture is quenched by the addition of silica followed by elution through a short silica column. The filtrate is concentrated in vacuo to give the desired product.
References
PubMed Litterature
Articles / Brochures
TCIMail
[Product Highlights] Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane[Product Highlights] A Bisphosphine Usable for Metal-free Hydrogenations
[Research Articles] High Throughput Sequence-controlled Oligosiloxane Synthesis
[Research Articles] Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)
[Research Articles] Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)
[Research Articles] Metal-Free Hydrogenation of N-Phenyl Aromatic Rings
[Research Articles] Metal-Free Hydrogenation of Imines Catalyzed by B(C6F5)3
Documents de produit (Note : Pour certains produits, les tableaux analytiques ne sont pas disponibles.)
Fiche de sécurité (FDS)
S'il vous plaît sélectionnez la langue.
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Veuillez remplir le numéro de lot
Le numéro de lot saisi est incorrect. Veuillez saisir uniquement 4-5 caractères alphanumériques avant le trait d'union.
Exemple de CoA
Il s'agit d'un échantillon CoA qui peut ne pas représenter un lot récemment fabriqué du produit.
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Veuillez remplir le numéro de lot
Le numéro de lot saisi est incorrect. Veuillez saisir uniquement 4-5 caractères alphanumériques avant le trait d'union.
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.





