Copper-mediated coupling reactions have been known as a traditional reaction. For instance, homocouplings of aryl halides and alkynes are called the Ullmann reaction and the Glaser coupling respectively, and both of these were discovered over a hundred years ago. Around the same time, carbon-heteroatom bond forming reactions such as the Ullmann ether synthesis and the Goldberg amination were also reported. In early studies of these classical copper-mediated coupling reactions, there were a number of disadvantages needing improvement such as use of over stoichiometric amounts of copper reagents, harsh reaction conditions, and low substrate generality. However, copper-mediated coupling reactions can be carried out at a lower cost compared with using palladium catalysts. So, various modified methods have been studied and more practical reactions have been developed.
Recent advances in the Ullmann-type reactions using aryl halides have been achieved under milder reaction conditions and reducing the amount of copper catalyst by the choice of suitable solvents, bases, and ligands. Also, investigation of effective ligands for these reactions has been developed and found that the diamine and dicarbonyl compounds effectively play as a ligand in preventing side reactions and deactivating a monovalent copper catalyst.
Maintenance Notice (4:30 AM - 11:00 AM December 21, 2024): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197




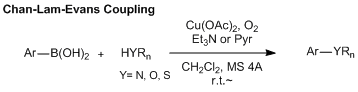










![Di-μ-hydroxo-bis[(N,N,N',N'-tetramethylethylenediamine)copper(II)] Chloride Di-μ-hydroxo-bis[(N,N,N',N'-tetramethylethylenediamine)copper(II)] Chloride](/medias/D2542.jpg?context=bWFzdGVyfHJvb3R8NDAwMTR8aW1hZ2UvanBlZ3xhR1V6TDJnd09DODRPVE13TURZek56UTFNRFUwTDBReU5UUXlMbXB3Wnd8N2Y2NjkxMDQxMWNkNjUyMDdjYTEyNjE4MzlkZWY5NzE5YmYyZGIxODRlN2M1OGYzMmViYjUwMzIzZjc5ZjhmYQ)
