Maximum quantity allowed is 999
Please select the quantity
A Valuable Synthetic Intermediate for 2-Substituted Adenosine
No.148(August 2012)
Adenosine derivatives play important roles in metabolic actions in various cells, and show antiviral and anticancer activities. 2-Iodoadenosine (1) is a valuable synthetic intermediate for the preparation of 2-substituted adenosine derivatives. Here are two typical adenosine derivatives syntheses using cross-coupling reactions. Matsuda et al. have reported a synthesis of 2-alkynyladenosines using a palladium-catalyzed cross-coupling reaction, and evaluation of their pharmacological activities.1) Zhao and Baranger have reported a synthesis of 2-alkenyladenosines using the Heck reaction.2)
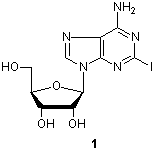
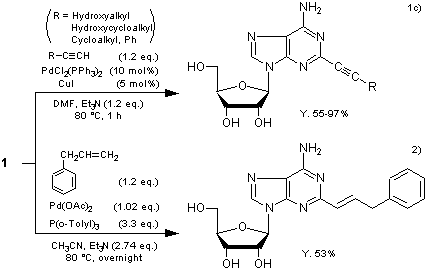
Typical Procedure1c):
2-Iodoadenosine (393 mg), CuI (9.5 mg), bis(triphenylphosphine)palladium(II) dichloride (36 mg), triethylamine (0.16 mL) and ethynylbenzene (123 mg) in DMF (7 mL) are heated at 80 °C for 1 h. After 2-iodoadenosine is completely consumed, the reaction mixture is concentrated to dryness under reduced pressure. The residue is dissolved in CHCl3, and H2S gas is introduced to the solution (~30 s) followed by N2 gas. The suspension is filtered through a Celite pad and washed with CHCl3. The combined filtrate is concentrated to dryness in vacuo, and the residue is purified by silica gel column chromatography (eluent: MeOH–CHCl3) to give 2-(phenylethyn-1-yl)adenosine (373 mg, Y. 97%).
2-Iodoadenosine (393 mg), CuI (9.5 mg), bis(triphenylphosphine)palladium(II) dichloride (36 mg), triethylamine (0.16 mL) and ethynylbenzene (123 mg) in DMF (7 mL) are heated at 80 °C for 1 h. After 2-iodoadenosine is completely consumed, the reaction mixture is concentrated to dryness under reduced pressure. The residue is dissolved in CHCl3, and H2S gas is introduced to the solution (~30 s) followed by N2 gas. The suspension is filtered through a Celite pad and washed with CHCl3. The combined filtrate is concentrated to dryness in vacuo, and the residue is purified by silica gel column chromatography (eluent: MeOH–CHCl3) to give 2-(phenylethyn-1-yl)adenosine (373 mg, Y. 97%).
References
- 1)Cross-coupling reaction with terminal alkynes
- 2)Cross-coupling reaction with terminal alkenes (Heck reaction)
Related Compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.