Maximum quantity allowed is 999
Please select the quantity
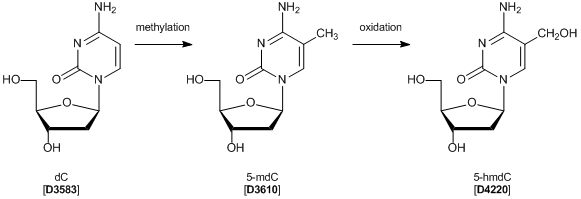
The Sixth Nucleoside of the Genome, 5-hmdC
2'-Deoxy-5-(hydroxymethyl)cytidine (5-hmdC) and its base 5-hydroxymethylcytosine (5-hmC) were recently discovered as oxidation products of 2’-deoxy-5-methylcytidine (5-mdC) in mammalian DNA, particularly in stem cell DNA. 5-mdC, a C5-methylated form of 2'-deoxycytidine (dC), has been studied as an indicator of DNA methylation in epigenetics. On the other hand, the biological function of 5-hmdC is currently not clear, but it is assumed that in stem cells it might be an intermediate of an active de-methylation process. Detection of 5-hmdC in a genome has been investigated to elucidate its role.
References
- The Discovery of 5-Formylcytosine in Embryonic Stem Cell DNA
- 5-Hydroxymethylcytosine, the Sixth Base of the Genome (Minireview)
- 5-Hydroxymethylcytosine Is Strongly Depleted in Human Cancers but Its Levels Do Not Correlate with IDH1 Mutations
- Determination of genomic 5-hydroxymethyl-2'-deoxycytidine in human DNA by capillary electrophoresis with laser-induced fluorescence
- Discrimination between 5-hydroxymethylcytosine and 5-methylcytosine in DNA by selective chemical labeling
- Deamination, Oxidation, and C–C Bond Cleavage Reactivity of 5-Hydroxymethylcytosine, 5-Formylcytosine, and 5-Carboxycytosine