Maintenance Notice (1:30 AM July 12 - 2:00 AM July 14, 2025 UTC): This website is scheduled to be unavailable due to maintenance. Maintenance may be completed earlier than scheduled. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News July 2025 | [TCIPracticalExample] Synthesis of a Carbamate... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
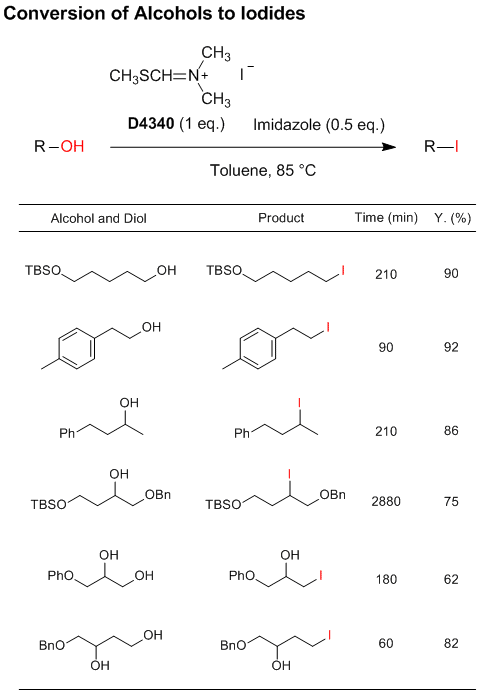
Novel Selective Iodinating Reagent
N,N-Dimethyl-N-(methylsulfanylmethylene)ammonium
iodide developed by Porter et al. is a novel selective iodinating reagent, which can convert primary
and secondary alcohols to the corresponding iodo compounds. In particular, primary alcohols react more rapidly
than secondary alcohols, which allows selective iodination of primary hydroxy groups or in primary-secondary
diols.
References
- Selective Conversion of Alcohols into Alkyl Iodides Using a Thioiminium Salt