Maximum quantity allowed is 999
CAS RN: 4525-65-9 | 제품번호: T3121
2,4,6-Trichlorophenyl Formate
순도/분석 방법: >97.0%(HPLC)
- Formic Acid 2,4,6-Trichlorophenyl Ester
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
| Purity(Argentometric Titration) | min. 95.0 % |
| Melting point | 81.0 to 85.0 °C |
| mp | 83 °C |
| 용해성 (용해) | Toluene |
| HS 번호* | 2915.13-000 |

-
Used Chemicals
-
Procedure
-
A solution of 2-iodofluorene (500 mg, 1.71 mmol), 2,4,6-trichlorophenyl formate (579 mg, 2.57 mmol), palladium(II) acetate (38.4 mg, 0.171 mmol), Xantphos (198 mg, 0.342 mmol) and triethylamine (470 µL, 3.42 mmol) in toluene (10 mL) was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure and the residue was washed with acetonitrile to give 1 as a pale yellow solid (462 mg, 69% yield).
A solution of 1 (460 mg, 1.18 mmol), triethylamine (330 µL, 2.36 mmol), DMAP (7.2 mg, 59 µmol) and morpholine (154 µL, 1.77 mmol) in THF (5 mL) was stirred at 45 °C for 20 hours. The reaction was quenched with water and the aqueous layer was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (ethyl acetate:hexane = 0:100 - 50:50) to give 2 as a white solid (305 mg, 93% yield).
-
Experimenter’s Comments
-
The reaction mixture at the first step was monitored by TLC (hexane:ethyl acetate = 1:19, Rf = 0.34).
The reaction mixture at the second step was monitored by TLC (hexane:ethyl acetate = 1:1, Rf = 0.26).
-
Analytical Data
-
2,4,6-Trichlorophenyl 9H-Fluorene-2-carboxylate (1)
(9H-Fluoren-2-yl)(morpholino)methanone (2)1H NMR (400 MHz, CDCl3);
(1) δ 8.41 (s, 1H), 8.29 (d, J = 8.1 Hz, 1H), 7.94-7.88 (m, 2H), 7.62 (d, J = 6.2 Hz, 1H), 7.48–7.38 (m, 4H), 4.02 (s, 2H).
(2) δ 7.81 (d, J = 7.6 Hz, 2H), 7.62-7.56 (m, 2H), 7.43-7.32 (m, 3H), 3.94 (s, 2H), 3.72 (brs, 8H). -
-
Lead Reference
-
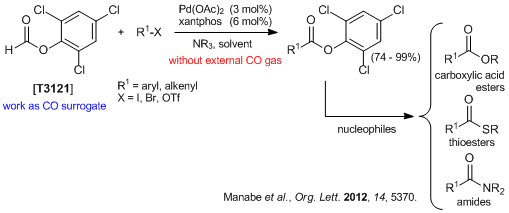
- Trichlorophenyl Formate: Highly Reactive and Easily Accessible Crystalline CO Surrogate for Palladium-Catalyzed Carbonylation of Aryl/Alkenyl Halides and Triflates.
-
Other References
-
- Pd-Catalyzed External-CO-Free Carbonylation: Preparation of 2,4,6-Trichlorophenyl 3,4-Dihydronaphthalene-2-Carboxylate
- Formic Acid Derivatives as Practical Carbon Monoxide Surrogates for Metal-Catalyzed Carbonylation Reactions
References
- 1)T. Ueda, H. Konishi, K. Manabe, Org. Lett. 2012, 14, 5370.

- 2)H. Konishi, T. Ueda, K. Manabe, Org. Synth. 2014, 91, 39.

- 3)H. Konishi, K. Manabe, Synlett 2014, 25, 1971.

[TCI Practical Example] Introduction of Carbonyl Group by Using 2,4,6-Trichlorophenyl Formate






