Maximum quantity allowed is 999
Please select the quantity
CAS RN: 14024-18-1 | 제품번호: I0079
Tris(2,4-pentanedionato)iron(III)
순도/분석 방법: >98.0%(T)
동의어의:
- Acetylacetone Iron(III) Salt
- Ferric(III) Acetylacetonate
- Iron(III) Acetylacetonate
제품문서:
•본 제품의 재고는 일본 본사의 재고입니다. 주문시, 5일안에 실험실까지 도착됩니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
규격표
| Appearance | Red to Dark red to Brown powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
물성치(참고치)
| mp | 185 °C(dec.) |
| Solubility in water | Slightly soluble |
| 용해성 (용해) | Acetone, dichloromethane, Benzene, Toluene, Ethanol, Chloroform |
GHS
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H302 : Harmful if swallowed. H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 주의 사항 | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. |
법규 정보
| RTECS # | NO8960000 |
운송 정보
| HS 번호* | 2914.40-000 |
Application
2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation

Reference
- Iron-Catalyzed Di?uoromethylation of Arylzincs with Di?uoromethyl 2?Pyridyl Sulfone
Application
Iron/dppbz-catalyzed Decarboxylative Couplings of Active Esters
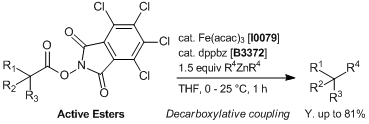
In a screwed-capped vial active ester (0.1 mmol), Fe(acac)3 (0.005 – 0.04 mmol) and dppbz (0.006 – 0.048 mmol) are weighed. The vial is then sealed, evacuated and back-filled with Ar. Then, 0.5 mL of distilled THF is added. The mixture is stirred for 5 minutes under Ar and diarylzinc reagent in THF (ca. 0.3 mol/L, 0.15 – 0.25 mmol) is added in one portion at 0 - 25 °C to the mixture and stirred at the same temperature for 1 h. After this time, the reaction is quenched with 1 mol/L HClaq, sat. NH4Claq or H2O and diluted with diethyl ether. The organic layer is separated and dried over Na2SO4 anhydrous, filtered and evaporated to dryness. Pure products are obtained after column chromatography or preparative TLC.
References
Application
Iron-Catalyzed ortho-Alkylation of Carboxamides

Typical procedure (R = benzyl): To Fe(acac)3 (17.1 mg, 0.05 mmol), dppe (29.9 mg, 0.075 mmol), and 3-methyl-N-(quinolin-8-yl)benzamide (131 mg, 0.5 mmol) are added THF (4 mL), and benzyl chloride (190 mg, 1.5 mmol). The mixture is then placed in an oil bath at 65 °C and PhMgBr (16% in THF, ca. 1 mol/L) (0.6 mL, 0.6 mmol) is added in a single portion. Upon completion of PhMgBr addition, the reaction mixture is stirred at 65 °C for 1 min. Then PhMgBr (16% in THF, ca. 1 mol/L) (1.3 mL, 1.3 mmol) is added dropwise at 65 °C for 9 min. The reaction mixture is stirred at 65 °C for 1 min and brought to rt. Subsequently the reaction mixture is quenched with a saturated solution of ammonium chloride (535 mg, 0.01 mol) and extracted with dichloromethane (3 x 3 mL). The combined organic extracts are dried over MgSO4, filtered and concentrated to give a residue. The residue is purified by silica gel flash chromatography (eluent: hexanes/EtOAc = 20:1) to give 2-benzyl-5-methyl-N-(quinolin-8-yl)benzamide (175 mg, 99% yield) as a white solid.
References
Application
Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
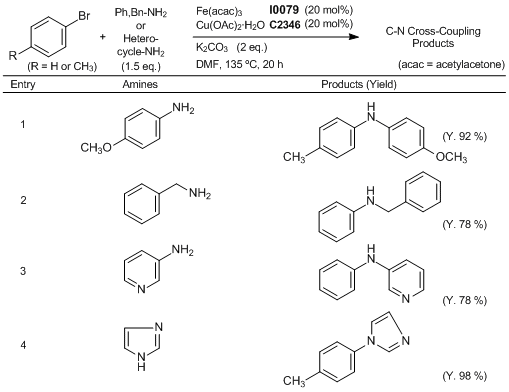
Typical procedure (Entry 1): A Schlenk tube is charged with p-anisidine (92.4 mg), K2CO3 (138 mg), Fe(acac)3 (34.4 mg), and Cu(OAc)2· H2O (20 mg). Under a nitrogen atmosphere, 4-bromotoluene (85.5 mg) is added followed by dry DMF (0.8 mL). The reaction mixture is then heated to 135 °C, and stirred for 20 h. The mixture is cooled to room temperature and diluted with diethyl ether. The resulting suspension is filtered through a celite pad and the filtrate is concentrated. The residue is purified by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to give 4-methoxy-N-p-tolylaniline (98.1 mg, 92 %).
References
Application
Iron-Catalyzed Cross-Coupling Reaction

Reference
PubMed Literature
문서
제품기사
[Product Highlights] 2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation[Research Articles] Fe or Ni-catalyzed Decarboxylative C-C Couplings of Active Esters
[Research Articles] Iron-Catalyzed ortho-Alkylation of Carboxamides
[Research Articles] Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
제품문서 (분석 차트를 제공하지 못할 수도 있으니 양해 바랍니다.)
SDS
언어를 선택하십시오.
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
샘플 시험성적서
시험성적서 샘플을 다운로드할 수 있습니다. 샘플은 반드시 최신 로트의 결과를 나타내는 것은 아닙니다.
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트

롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
죄송합니다만 찾으시는 분석차트는 없습니다.





