Maximum quantity allowed is 999
Please select the quantity
CAS RN: 1678540-23-2 | 제품번호: E1267
Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate
![Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate No-Image](/medias/E1267.jpg?context=bWFzdGVyfHJvb3R8NzE4MDJ8aW1hZ2UvanBlZ3xhREV6TDJnMVlTODRPVEl4TmpZek5qWTRNalUwTDBVeE1qWTNMbXB3Wnd8YTE2MGM4Y2FhYzFlMDNlYjEwYTk5NTg1NDYwOTc5ZmE1M2EwNDhiYTFiZjM5ZDlkY2VjYjE4ODM3MmM1OWJjYQ)
| 포장단위 | 가격 | 당일출고 | 2-3일 이내 출고 |
|---|---|---|---|
| 50MG |
₩473,600
|
2 | 문의 |
•본 제품의 재고는 일본 본사의 재고입니다. 주문시, 5일안에 실험실까지 도착됩니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.(SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.(SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | E1267 |
| Purity/Analysis Method | >97.0%(HPLC) |
| M.F. / M.W. | C__5__4H__6__3NO__2 = 758.10 |
| 물리적 상태 (20 ℃) | Solid |
| 보관 조건 | Refrigerated (0-10°C) |
| 피해야 할 조건 | Heat Sensitive |
| CAS RN | 1678540-23-2 |
| PubChem Substance ID | 354334205 |
규격표
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
물성치(참고치)
GHS
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 주의 사항 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
법규 정보
운송 정보
| HS 번호* | 2922.49-000 |
Application
Decarboxylative Asymmetric Chlorination Using an Asymmetric Catalyst

Experimental procedure:
To a stirred solution of 1 (10 mol%), N-chlorosuccinimide (1.5 eq.) in toluene is added α-alkyl-β-ketocarboxylic acid (1 eq.) and the reaction mixture is stirred under darkness. After completion, the resulting mixture is subjected directly silica gel column chromatography (hexane:dichloromethane) to give the corresponding α-chloroketone.
To a stirred solution of 1 (10 mol%), N-chlorosuccinimide (1.5 eq.) in toluene is added α-alkyl-β-ketocarboxylic acid (1 eq.) and the reaction mixture is stirred under darkness. After completion, the resulting mixture is subjected directly silica gel column chromatography (hexane:dichloromethane) to give the corresponding α-chloroketone.
References
- Enantioselective decarboxylative chlorination of β-ketocarboxylic acids
Application
An Enantioselective Fluorination of α-Branched Aldehydes Using Newly Developed Chiral Amine Catalyst
div class="blockC">
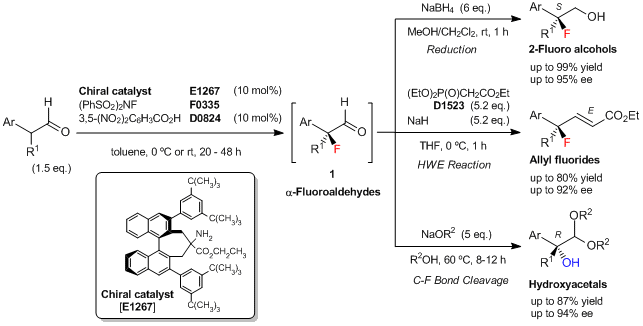
Typical Procedure (synthesis of 2-fluoro alcohol; Ar=Ph, R1=CH3):
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
References
PubMed Literature
기사 / 브로셔
TCIMail
[Research Articles] Decarboxylative Asymmetric Chlorination Using an Asymmetric Catalyst[Research Articles] An Enantioselective Fluorination of α-Branched Aldehydes Using Newly Developed Chiral Amine Catalyst
제품문서 (분석 차트를 제공하지 못할 수도 있으니 양해 바랍니다.)
SDS
언어를 선택하십시오.
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
샘플 시험성적서
시험성적서 샘플을 다운로드할 수 있습니다. 샘플은 반드시 최신 로트의 결과를 나타내는 것은 아닙니다.
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
죄송합니다만 찾으시는 분석차트는 없습니다.

![Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate Ethyl (11bR)-4-Amino-2,6-bis(3,5-di-tert-butylphenyl)-4,5-dihydro-3H-cyclohepta[1,2-a:7,6-a']dinaphthalene-4-carboxylate](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

