Maximum quantity allowed is 999
Please select the quantity
CAS RN: 52462-29-0 | 제품번호: D2751
Dichloro(p-cymene)ruthenium(II) Dimer

•본 제품의 재고는 일본 본사의 재고입니다. 주문시, 5일안에 실험실까지 도착됩니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | D2751 |
| Purity/Analysis Method | >95.0%(T) |
| M.F. / M.W. | C__2__0H__2__8Cl__4Ru__2 = 612.38 |
| 물리적 상태 (20 ℃) | Solid |
| 보관 조건 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 용기 | 1G-Glass Bottle with Plastic Insert (이미지 보기) |
| CAS RN | 52462-29-0 |
| PubChem Substance ID | 87569153 |
| MDL 번호 | MFCD00064793 |
규격표
| Appearance | Light yellow to Brown powder to crystal |
| Purity(Argentometric Titration) | min. 95.0 % |
물성치(참고치)
GHS
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. H412 : Harmful to aquatic life with long lasting effects. |
| 주의 사항 | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
법규 정보
운송 정보
| HS 번호* | 2843.90-000 |
Application
Ruthenium-catalyzed Direct C―H Amidation of Arenes

Typical Procedure:
Acetophenone (0.4 mmol), p-toluenesulfonyl azide (0.2 mmol), [RuCl2(p-cymene)]2 (4.9 mg, 4 mol%), AgNTf2 (12.5 mg, 16 mol%), NaOAc (3.3 mg, 20 mol%), and 1,2-dichloroethane (0.5 mL) are added to a screw-capped vial equipped with a spinvane triangular-shaped Teflon stirbar. The reaction mixture is stirred in a preheated oil bath at 80 °C for 12 h and then cooled to room temperature, filtered through a pad of Celite, and washed with ethyl acetate (10 mL×3). The filtrate is concentrated under reduced pressure and the residue is purified by chromatography on silica gel (n-hexane : EtOAc = 3 : 1) to give the desired product (56.1 mg, Y. 97%).
Acetophenone (0.4 mmol), p-toluenesulfonyl azide (0.2 mmol), [RuCl2(p-cymene)]2 (4.9 mg, 4 mol%), AgNTf2 (12.5 mg, 16 mol%), NaOAc (3.3 mg, 20 mol%), and 1,2-dichloroethane (0.5 mL) are added to a screw-capped vial equipped with a spinvane triangular-shaped Teflon stirbar. The reaction mixture is stirred in a preheated oil bath at 80 °C for 12 h and then cooled to room temperature, filtered through a pad of Celite, and washed with ethyl acetate (10 mL×3). The filtrate is concentrated under reduced pressure and the residue is purified by chromatography on silica gel (n-hexane : EtOAc = 3 : 1) to give the desired product (56.1 mg, Y. 97%).
References
Application
Ruthenium-Catalyzed Isoquinolone Synthesis
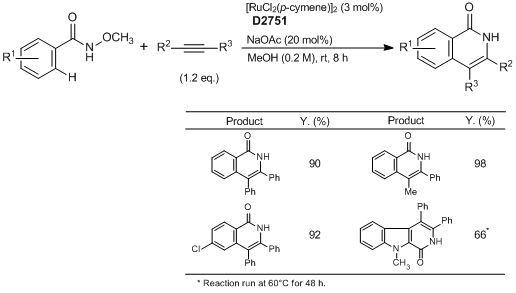
Typical Procedure:
A mixture of N-methoxybenzamide (0.50 mmol, 1.0 eq.), the alkyne (if solid) (0.60 mmol, 1.2 eq.), [RuCl2(p-cymene)]2 (9.2 mg, 0.015 mmol, 3.0 mol%) and NaOAc (8.2 mg, 0.10 mmol, 20.0 mol%) are placed in a Schlenk tube equipped with a stir bar. Dry MeOH (2.5 mL) is added (followed by the alkyne if it is a liquid) and the mixture is stirred at room temperature for 8 h under an argon atmosphere. Afterwards, the mixture is diluted with CH2Cl2 and transferred to a round-bottomed flask. Silica is added to the flask and volatiles are evaporated under reduced pressure. The purification is performed by flash column chromatography on silica gel (EtOAc : petroleum ether=1:5 to 1:1, typically) to give the corresponding pure products.
A mixture of N-methoxybenzamide (0.50 mmol, 1.0 eq.), the alkyne (if solid) (0.60 mmol, 1.2 eq.), [RuCl2(p-cymene)]2 (9.2 mg, 0.015 mmol, 3.0 mol%) and NaOAc (8.2 mg, 0.10 mmol, 20.0 mol%) are placed in a Schlenk tube equipped with a stir bar. Dry MeOH (2.5 mL) is added (followed by the alkyne if it is a liquid) and the mixture is stirred at room temperature for 8 h under an argon atmosphere. Afterwards, the mixture is diluted with CH2Cl2 and transferred to a round-bottomed flask. Silica is added to the flask and volatiles are evaporated under reduced pressure. The purification is performed by flash column chromatography on silica gel (EtOAc : petroleum ether=1:5 to 1:1, typically) to give the corresponding pure products.
References
PubMed Literature
기사 / 브로셔
TCIMail
[TCIMAIL No.107] Arene Ruthenium(II) Complexes[Research Articles] Ruthenium-catalyzed Direct C-H Amidation of Arenes
[Research Articles] Ruthenium-Catalyzed Isoquinolone Synthesis
제품문서 (분석 차트를 제공하지 못할 수도 있으니 양해 바랍니다.)
SDS
언어를 선택하십시오.
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
샘플 시험성적서
시험성적서 샘플을 다운로드할 수 있습니다. 샘플은 반드시 최신 로트의 결과를 나타내는 것은 아닙니다.
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
죄송합니다만 찾으시는 분석차트는 없습니다.




