Maximum quantity allowed is 999
CAS RN: 12148-71-9 | 제품번호: C2662
(1,5-Cyclooctadiene)(methoxy)iridium(I) Dimer
순도/분석 방법:
- Bis(1,5-cyclooctadiene)di-μ-methoxydiiridium(I)
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | C2662 |
| M.F. / M.W. | C__1__8H__3__0Ir__2O__2 = 662.87 |
| 물리적 상태 (20 ℃) | Solid |
보관 조건 
|
Frozen (<0°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Light Sensitive,Air Sensitive,Moisture Sensitive,Heat Sensitive |
용기 
|
1G-Glass Bottle with Plastic Insert (이미지 보기), 200MG-Glass Bottle with Plastic Insert (이미지 보기) |
| CAS RN | 12148-71-9 |
| Reaxys-RN | 14520157 |
| PubChem Substance ID | 160871404 |
| MDL 번호 | MFCD08459360 |
| Appearance | Light yellow to Amber to Dark green powder to crystal |
| Elemental analysis(Carbon) | 31.50 to 34.00 % |
| mp | 179 °C(dec.) |
| HS 번호* | 2843.90-000 |
-
Used Chemicals
-
Procedure
-
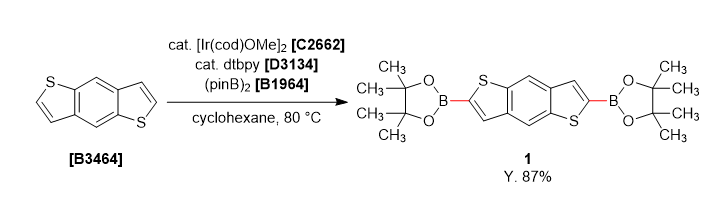
A solution of dtbpy (27 mg, 0.05 mmol), bis(pinacolato)diboron (1.0 g, 4.0 mmol) and [Ir(cod)OMe]2 (33 mg, 0.025 mmol) in cyclohexane (40 mL) was stirred under nitrogen at room temperature for 10 min. Benzo[1,2-b:4,5-b']dithiophene (380 mg, 2.0 mmol) was added the mixture and stirred 80 ˚C for 18 hours. The reaction mixture was quenched with water and separated both layers, extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the crude was washed with methanol (20 mL) to give 1 as a white solid (0.771 g, 87% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NMR.
Cyclohexane was bubbled with nitrogen before use.
-
Analytical Data
-
Compound 1
1H NMR (270 MHz, CDCl3); δ 8.36 (s, 2H), 7.90 (s, 2H), 1.39 (s, 24H).
-
Lead Reference
-
- Synthesis and Transistor Application of Bis[1]benzothieno[6,7‑d:6′,7′‑d′]benzo[1,2‑b:4,5‑b′]dithiophenes

Reference
- Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent

An alcohol or ketone substrate is dissolved in THF and treated with a freshly prepared solution of [Ir(cod)OMe]2 (0.05 mol%) in THF and then with neat Et2SiH2 (1.2 eq.). The resulting solution is stirred at room temperature (23 °C) until complete conversion of the alcohol or ketone. At the completion of the reaction, the corresponding diethyl(hydrido)silyl ether is observed. Then the reaction mixture is placed under high vacuum for 1 h. The concentrated diethyl(hydrido)silyl ether is sequentially treated with freshly prepared solutions of norbornene (1.2 eq.) in THF and [Ir(cod)OMe]2 (0.5 mol%) in THF, and then with a slurry of Me4phen (1.25 mol%) in THF. The resulting solution is stirred at room temperature for 1 h and then heated it at 80-120 °C until complete conversion to the corresponding oxasilolane is observed. Then the crude reaction mixture containing the oxasilolane is sequentially treated with MeOH, KHCO3 (2.5 eq.) and H2O2 (30% solution in H2O, 10 eq.), and the resulting mixture is stirred overnight at 50 °C. The reaction is carefully quenched with aq. NaHSO3, and the resulting mixture is extracted with EtOAc. The combined organic layer is sequentially washed with 1 M HCl and sat. NaHCO3, and then dried with MgSO4. The resulting organic layer is filtered through Celite and concentrated to provide the crude diol, which is either purified directly or after conversion to the corresponding acetate derivative through treatment with Ac2O and Et3N.
References
[Research Articles] Catalytic Functionalization of Un-activated Primary C-H Bonds
SDS
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
샘플 시험성적서
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트

죄송합니다만 찾으시는 분석차트는 없습니다.





