Maximum quantity allowed is 999
CAS RN: 32005-36-0 | 제품번호: B1374
Bis(dibenzylideneacetone)palladium(0)

순도/분석 방법:
- Palladium(0) Bis(dibenzylideneacetone)
- Bis(dba)palladium(0)
- Pd(dba)2
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | B1374 |
| M.F. / M.W. | C__3__4H__2__8O__2Pd = 575.02 |
| 물리적 상태 (20 ℃) | Solid |
보관 조건 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Air Sensitive |
용기 
|
1G-Glass Bottle with Plastic Insert (이미지 보기) |
| CAS RN | 32005-36-0 |
| PubChem Substance ID | 87564299 |
| SDBS | 50493 |
| MDL 번호 | MFCD00051942 |
| Appearance | Brown to Black powder to crystal |
| Content (Palladium) | 16.5 to 20.5 % |
| mp | 150 °C |
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H302 + H312 + H332 : Harmful if swallowed, in contact with skin or if inhaled. H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 주의 사항 | P501 : Dispose of contents/ container to an approved waste disposal plant. P261 : Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. P270 : Do not eat, drink or smoke when using this product. P271 : Use only outdoors or in a well-ventilated area. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. P301 + P312 + P330 : IF SWALLOWED: Call a POISON CENTER/doctor if you feel unwell. Rinse mouth. P302 + P352 + P312 : IF ON SKIN: Wash with plenty of water.Call a POISON CENTER/doctor if you feel unwell. P304 + P340 + P312 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/doctor if you feel unwell. |
| HS 번호* | 2843.90-000 |
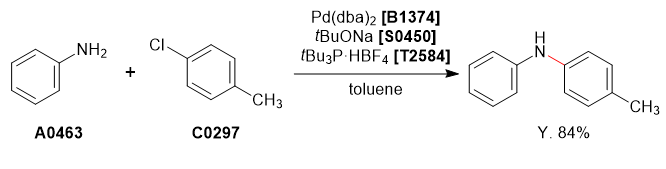
Used Chemicals
Procedure
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Experimenter’s Comments
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
Analytical Data (4-Methyl-N-phenylaniline)
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).
Lead Reference
- Tetramethoxybenzene is a Good Building Block for Molecular Wires: Insights from Photoinduced Electron Transfer
Used Chemicals
- 2-Chloro-m-xylene [C2164]
- 2-Methylphenylboronic Acid [M1313]
- Bis(dibenzylideneacetone)palladium (0) (Pd(dba)2) [B1374]
- 2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) [D5036]
- Tripotassium Phosphate (K3PO4)
- Toluene
- Ion-exchange water
Procedure
To a 3-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (66 mg, 0.115 mmol, 1.5 mol%), SPhos (94 mg, 0.229 mmol, 3.0 mol%), 2-methylphenylboronic acid (1.56 g, 11.5 mmol, 1.5 equiv.), tripotassium phosphate (4.87 g, 22.9 mmol, 3.0 equiv.) degassed toluene (15 mL), and ion-exchange water (1.5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 2-Chloro-m-xylene (1.0 mL, 7.64 mmol, 1.0 equiv.) was added one portion. The resulting mixture was stirred at reflux for 7 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane only) to afford the corresponding compound as a colorless oil (1.46 g, 97%).
Experimenter's Comments
i) Toluene was degassed by bubbling with nitrogen gas for 30 min.
ii) The reaction mixture was monitored by GC.Analytical Data(2,2',6-Trimethyl-1,1'-biphenyl)
1H NMR (400 MHz, CDCl3); δ 7.23-7.33 (m, 3H), 7.22-7.10 (m, 3H), 7.00-7.06 (m, 1H), 1.99 (s, 3H), 1.97 (s, 6H).
13C NMR (101 MHz, CDCl3); δ 141.0, 140.5, 135.8, 135.5, 129.9, 128.8, 127.2 127.0, 126.9, 126.0, 20.3, 19.4.
Lead Reference
- Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure
Used Chemicals
Procedure
-
To a 2-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (36 mg, 0.0633 mmol, 1.5 mol%), XPhos (60 mg, 0.127 mmol, 3.0 mol%), sodium tert-butoxide (811 mg, 8.44 mmol, 2.0 equiv.) and toluene (5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 4-chlorotoluene (0.5 mL, 4.22 mmol, 1.0 equiv.), and morpholine (0.55 mL, 6.33 mmol, 1.5 equiv.) were added in one portion. The resulting mixture was stirred at reflux for 6 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane : ethyl acetate = 9 : 1) to afford the corresponding compound as an orange solid (700 mg, 94%).
Experimenter's Comments
Toluene was degassed by bubbling with nitrogen gas for 30 min.
The reaction mixture was monitored by GC.Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.10 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 3.87 (t, J = 4.8 Hz, 4H), 3.11 (t, J = 4.8 Hz, 4H), 2.28 (s, 3H).
13C NMR (101 MHz, CDCl3); δ 149.1, 129.7, 129.5, 116.0, 66.9, 49.9, 20.4.
Lead Reference
- Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions
- Pd-Catalyzed Kumada-Corriu Cross-Coupling Reactions at Low Temperatures Allow the Use of Knochel-type Grignard Reagents


References
문서
SDS
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
샘플 시험성적서
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트

죄송합니다만 찾으시는 분석차트는 없습니다.





![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)


