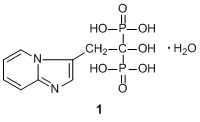
Bisphosphonates (BPs), including minodronate, ibandronate, etc., are very effective inhibitors of bone resorption in vivo and in vitro. Minodronate monohydrate (1) is one of BPs.1,2) These compounds are characterized by two C-P bonds which are located on the same carbon atom, i.e. geminal BPs, and have been used for many research studies regarding bone metabolism.1,2)
According to differences in the side chain, BPs can be divided into two groups, non-nitrogen containing BPs (non-N-BPs) and nitrogen-containing BPs (N-BPs). 1 is a N-BP and is classified as a third-generation heterocyclic N-BP.2,3) N-BPs inhibit farnesyl pyrophosphate synthetase, a key enzyme in the mevalonate pathway, which affects cellular activity and survival.1,2) Regarding inhibition of bone resorption, 1, as well as zoledronic acid, is more effective than other BPs.4)
Maximum quantity allowed is 999
Please select the quantity
Bone Resorption Inhibitor
No.160(January 2014)
References
- 1)Bisphosphonates from bench to bedside
- 2)Computational insights into binding of bisphosphonates to farnesyl pyrophosphate synthase
- 3)A review of minodronic acid hydrate for the treatment of osteoporosis
- 4) Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.