Maximum quantity allowed is 999
Please select the quantity
Pincer Type Rh Complex Catalyzed Asymmetric Diboration of Terminal Alkenes and Their Conversion to the Optically Active 1,2-Diols
No.160(January 2014)
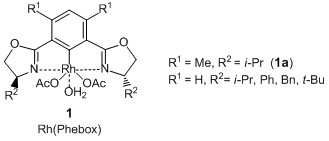

Nishiyama et al. have reported that optically active 1,2-diols are given by the pincer type Rh complexes [Rh(Phebox), 1] catalyzed asymmetric 1,2-diboration of terminal alkenes and subsequent oxidation. According to their results, the asymmetric diboration of 4-chlorostyrene with a diboron ester proceeds with high enantioselectivity by using a rhodium complex coordinated with a pincer type ligand (1a), and the desired 1,2-diborylated product is formed. Subsequent oxidation of it with sodium peroxoborate in THF-water gives the corresponding 1,2-diol. In this reaction, allyl ethers and allyl anilines can be transformed into the related optically active 1,2-diols. In addition, when using trans 1,3-dienes as reactants, terminal alkene moieties are selectively reacted and the optically active allylic alcohols are given. Thus, this asymmetric diboration using 1 is an efficient synthetic method to produce the optically active 1,2-diols.
References
- Asymmetric diboration of terminal alkenes with a rhodium catalyst and subsequent oxidation: enantioselective synthesis of optically active 1,2-diols
Related Compounds
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.