Maintenance Notice (10:30 PM June 7 - 2:00 AM June 8, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News June 2025 | [Product Highlights] Benzoquinone Derivative Useful for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
An Efficient Chiral Organocatalyst for the Cross-aldol Reaction of Aliphatic Ketones with Aldehydes
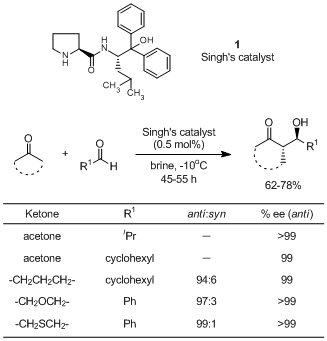
A proline derived chiral organocatalyst, 1, has been developed by Singh and used for chiral cross-aldol reactions in brine. 1-catalyzed direct cross-aldol reactions are widely applicable to various substances such as aliphatic ketones and α-branched aliphatic aldehydes and afford the desired cross-aldol adducts with high enantioselectivities though it is commonly considered to be difficult to use them for direct cross-aldol reactions. Furthermore, using DMF as a solvent, direct cross-aldol reactions of isobutyraldehyde with linear aldehydes successfully proceed to give the cross-aldol adducts enantioselectively. It is assumed that 1 activates the formyl group by hydrogen bonding with both the NH and OH of the amino alcohol moiety while the two phenyl groups of the catalyst 1 induce the stereoselectivity.
References
- Highly Efficient Small Organic Molecules for Enantioselective Direct Aldol Reaction in Organic and Aqueous Media
- Highly Enantioselective Organocatalytic Direct Aldol Reaction in an Aqueous Medium
- Highly Enantioselective Direct Aldol Reaction Catalyzed by Organic Molecules
- Enantioselective Aldol Reactions of Aliphatic Aldehydes with Singh’s Catalyst