Maintenance Notice (10:30 PM June 7 - 2:00 AM June 8, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Product Document Searching Made Easy by 2D Code! | TCI Chemistry News June 2025 | [Product Highlights] Benzoquinone Derivative Useful for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
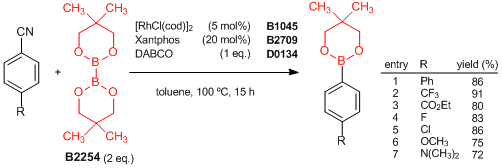
Rhodium(I)-Catalyzed Aryl Nitrile Borylation through the Cleavage of Carbon-Cyano Bonds
Tobisu et al. have reported a rhodium(I)-catalyzed borylation of aryl nitriles using bis(neopentyl glycolato)diboron. The reaction of aryl nitriles with the diboron in the presence of the rhodium/Xantphos catalyst and DABCO affords these arylboronic esters, even when there are other functional groups (R), via carbon-cyano bond cleavage. The carbon-cyano bond is not cleaved under typical transition-metal-catalyzed conditions, which allows the regioselective coupling to be combined with other transition-metal-catalyzed couplings. Therefore, this reaction would be a new strategy for the synthesis of complex boronic acid derivatives.