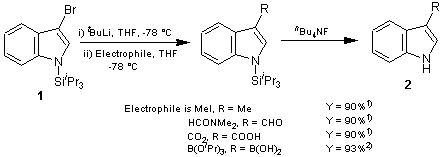
The 3-lithiated indole is generated from silylindole 1 by treatment with alkyllithium and when allowed to react at -78°C with various electrophiles, regioselectively yields 3-substituted indoles in good yield . This is because the lithiated intermediate contains a bulky triisopropylsilyl group at the 1-position. The steric bulk provides protection at the 2-position of indole which prevent 3→2 migration of lithium and subsequent ring fragmentation1).
Reported applications of 1 are the synthesis of tryptophan2) and the total synthesis of grossularine-13) which is a type of alkaloid.
Maximum quantity allowed is 999
Please select the quantity
Synthesis of 3-Substituted Indoles
No.105(June 2001)
References
- 1)Synthesis of 3-substituted indoles
- 2)Synthesis of β-Methyltryptophan
- 3)Total synthesis of Grossularines-1 and -2
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
