Maximum quantity allowed is 999
CAS RN: 13675-18-8 | Product Number: T2953
Tetrahydroxydiboron

Purity:
- Tetrahydroxydiborane
- Diboronic Acid
- Hypodiboric Acid
- BBA
- Bis-Boric Acid
* The displayed price is the unit price and does not include consumption tax. The unit prices displayed are the latest and are subject to change without notice.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Appearance | White to Almost white powder to crystal |
| Purity(Neutralization titration) | 98.0 to 110.0 % |
| Melting Point | 290 °C |
| Solubility (soluble in) | Methanol |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| PRTR Law (New Specific Chemical) | Class 1 Designated Chemical Substances |

-
Used Chemicals
-
Procedure
-
To a solution of trans-4-nitrostilbene (1.13 g, 5.00 mmol), diboronic acid (1.34 g, 15.0 mmol, 3.0 eq.) in DMF (30 mL) was added 4,4’-bipyridyl (3.9 mg, 0.025 mmol, 0.50 mol%) at r.t. and the mixture was stirred at the same temperature for 1 hour. The reaction mixture was diluted by 1 mol/L NaHCO3 aq. (100 mL) and the resulting solid was filtered off. The solid was purified by column chromatography (dichloromethane:hexane = 0:1 - 4:1 on silica gel) to give trans-4-aminostilbene as a yellow solid (891 mg, y. 91%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by UPLC.
-
Analytical Data
-
trans-4-Aminostilbene
1H NMR (270 MHz, CDCl3); δ 7.47 (d, 2H, J = 7.0 Hz), 7.38-7.30 (m, 4H), 7.21 (t, 1H, J = 7.0 Hz), 7.03 (d, 1H, J = 15 Hz), 6.91 (d, 1H, J = 15 Hz), 6.63 (d, 2H, J = 8.6 Hz), 3.75 (brs, 2H).
-
Lead Reference
-
- Metal-Free, Rapid, and Highly Chemoselective Reduction of Aromatic Nitro Compounds at Room Temperature
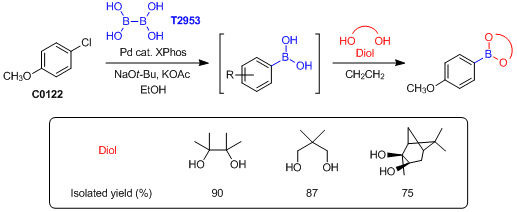
To an oven-dried glass vessel is added (X-Phos) palladium(II) phenethylamine chloride (7.38 mg, 0.01 mmol), X-Phos (9.52 mg, 0.02 mmol), tetrahydroxydiborane, (133.5 mg, 1.5 mmol), KOAc (294 mg, 3 mmol), and NaOt-Bu (1 mg, 0.01 mmol). The vessel is evacuated and backfilled with N2 (three times). EtOH (10 mL degassed) is added followed by the addition of 4-chloroanisole (1 mmol). The reaction is then heated to 80 °C for 18 hours. The reaction is cooled to rt then filtered through a thin pad of Celite (eluting with EtOAc) and concentrated. To the residue is added 1 M aqueous HCl and EtOAc (25 mL each). This mixture is stirred 30 min and the aqueous layer is removed, and the organic layer is washed with brine. The combined aqueous layers are extracted with EtOAc (3 x 10 mL). The combined organics are dried (Na2SO4) and then concentrated. The crude mixture is taken up in CH2Cl2, the corresponding diol is added (1.35 mmol), and the crude reaction is allowed to stir at rt. At completion of the reaction, the reaction is concentrated, and the residue is purified by flash column chromatography to afford the desired aryl boric acid derivatives.
References
- 1)Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives
Articles/Brochures
[Product Highlights] Diboronic Acid Usable for the Direct Synthesis of Aromatic Boric Acid Derivatives
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.





