Maximum quantity allowed is 999
CAS RN: 258516-84-6 | Product Number: D5828
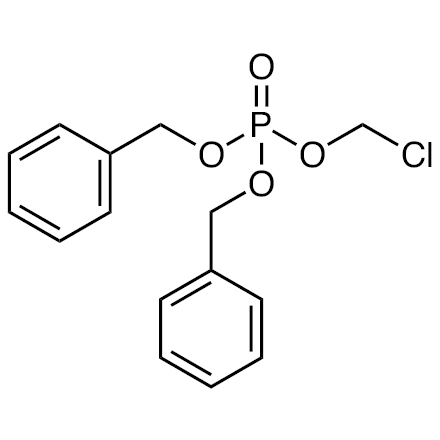
Dibenzyl (Chloromethyl) Phosphate
Purity: >95.0%(HPLC)
| Size | Unit Price | Saitama (Kawaguchi) | Hyogo (Amagasaki) | Stock in other WH |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
¥40,200
|
18 | 2 | Contact Us |
* The displayed price is the unit price and does not include consumption tax. The unit prices displayed are the latest and are subject to change without notice.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | D5828 |
Purity / Analysis Method 
|
>95.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__1__5H__1__6ClO__4P = 326.71 |
| Physical State (20 deg.C) | Liquid |
Storage Temperature 
|
Refrigerated (0-10°C) |
| Condition to Avoid | Heat Sensitive |
| CAS RN | 258516-84-6 |
| Reaxys Registry Number | 9131198 |
| PubChem Substance ID | 468591588 |
| MDL Number | MFCD06652586 |
| Appearance | Colorless to Light orange to Yellow clear liquid |
| Purity(HPLC) | min. 95.0 area% |
| NMR | confirm to structure |
| Flash point | 346 °C |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |

Used Chemicals
Procedure
To a reaction vessel were added 2,6-diisopropylphenol (0.42 g, 2.36 mmol) and DMF (20 mL) under nitrogen atmosphere, cooling with an ice bath. Sodium hydride (60%, dispersed in liquid paraffin) (0.113 g, 2.83 mmol) was added and stirred for 30 min, then (chloromethyl)dibenzyl phosphate (1.0 g, 3.06 mmol) was added. The mixture was stirred for 2 h at room temperature and cooled with an ice bath. Water (20 mL) was added and extracted with diethyl ether. The organic layer was sequentially washed with water and brine, dried over magnesium sulfate, filtered and the filtrate was concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (hexane:ethyl acetate = 5:1) to give 1 (0.8 g, 72% yield) as a colorless liquid.
Experimenter’s Comments
The reaction mixture was monitored by TLC (hexane:ethyl acetate = 5:1, Rf = 0.3).
Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.38-7.27 (m, 10H), 7.17-7.09 (m, 3H), 5.42 (d, 2H, J = 9.6 Hz), 5.06-4.95 (m, 4H), 3.33(sep, 2H, J = 6.9 Hz), 1.18 (d, 12H, J = 6.9 Hz).
Lead Reference
- Synthesis and evaluation of prodrugs of corticotropin-releasing factor-1 (CRF1) receptor antagonist BMS-665053 leading to improved oral bioavailability
Lead Reference
- Synthesis and Characterization of a Phosphate Prodrug of Isoliquiritigenin
Documents
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts

The requested analytical chart is not available. Sorry for the inconvenience.