Maximum quantity allowed is 999
Please select the quantity
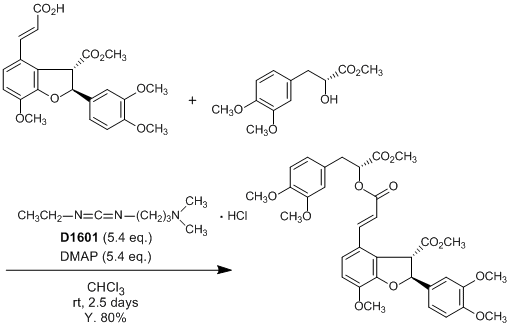
CAS RN: 25952-53-8 | Product Number: D1601
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride [Coupling Agent for Peptides Synthesis]
Purity: >98.0%(T)
Chemical Substance Law (ENCS):
2-1696
Synonyms:
- 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide Hydrochloride
- EDC·HCl
- EDAC·HCl
- EDCI·HCl
Product Documents:
* Available Stock: Prompt shipment (for products) in Saitama/Hyogo warehouse. Stock In Other WH: Shipment in about 2-3 Business Days from Saitama warehouse. Please contact us
if you need further information.
* The displayed price is the unit price and does not include consumption tax. The unit prices displayed are the latest and are subject to change without notice.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
* The displayed price is the unit price and does not include consumption tax. The unit prices displayed are the latest and are subject to change without notice.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | D1601 |
| Purity / Analysis Method | >98.0%(T) |
| Grade | SU |
| Molecular Formula / Molecular Weight | C8H17N3·HCl = 191.70 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive,Heat Sensitive |
| CAS RN | 25952-53-8 |
| Reaxys Registry Number | 5764110 |
| PubChem Substance ID | 87568083 |
| SDBS (AIST Spectral DB) | 21309 |
| MDL Number | MFCD00012503 |
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.

![1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride [Coupling Agent for Peptides Synthesis] No-Image](/medias/D1601.jpg?context=bWFzdGVyfHJvb3R8MzY1MzJ8aW1hZ2UvanBlZ3xhR00wTDJnME9DODRPVEl4TURVME5URXhNVE0wTDBReE5qQXhMbXB3Wnd8ZDQ1N2ExZWJmNWJjMTI0NDY1NjU2MWEyYWNkNzVhYTlmZGRlMGJhNDYzYWMzMDU3MmM0MzM0YmIwY2YxYWI0OA)

![1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride [Coupling Agent for Peptides Synthesis] 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride [Coupling Agent for Peptides Synthesis]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)