Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
CAS RN: | Product Number: C2637
Copper(II) on Chitosan (Cu 1.3mmol/g)
Purity:
Synonyms:
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 5G |
€250.00
|
1 | 10 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | C2637 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
Specifications
| Appearance | Green to Dark green to Dark blue powder to crystal |
| Loading | 1.2 to 1.5 mmol/g |
| Functionality test | to pass test |
Properties (reference)
GHS
Related Laws:
Transport Information:
| HS Number | 3822190090 |
Application
Copper (II) on Chitosan, a reagent for selective discrimination between 2-hydroxy and 2-deoxy sugars, an alternative test reagent for Fehling and Benedict reagents
Copper (II) on chitosan is a chitosan-supported copper (II) (about 1.3 mmol/g) developed by Inoue et al. as a reagent for selective detection of reducible organic compounds. The Cu (II) on chitosan can selectively detect reducible organic compounds bearing structures changeable to ene-diolate (e.g. glucose, ribose) in basic aqueous solution with the formation of copper (I) oxide (Cu2O) as a signal of the reduction by the ene-diolate.

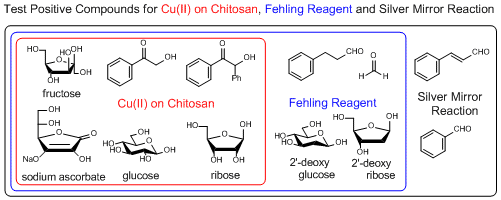
(The color of Cu (II) on chitosan is subject to change to green after opening. But the green-colored Cu (II) on chitosan changes to dark blue which is suitable for this detection in basic aqueous solution, in the same way as a new reagent.)
Typical procedure (detection of a sugar): A test tube (15 mm inside diameters) is charged with copper (II) on chitosan (20 mg) and an aqueous solution of 0.5 mol/L NaOH (1 mL). At this point, the color of copper (II) on chitosan changes from blue to dark blue. Then, an aqueous solution of 10 mmol/L sugar (4 mL) is added, and the mixture is stirred at 70 to 80 °C for 5 min.
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).
If it gives a positive result, red-brown to orange colored Cu2O on chitosan is observed (e.g. glucose and ribose). If it gives a negative result, black to blue colored CuO on chitosan is observed (e.g. 2-deoxyglucose and 2-deoxy ribose).

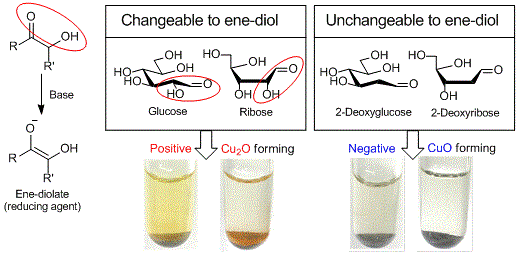
Using Cu (II) on chitosan, needs a lesser amount of Cu (II) than that of Fehling and Benedict reagents and leads to significantly reducing the reagent costs and waste chemicals. In addition, Cu (II) on chitosan is a powdery substance, which is easily handled and free from leaking in carrying and storage. Notably, Cu (II) on chitosan is suitable for science educational experiments such as a test for the detection of a reducing sugar yielded from starch hydrolysis.

References
- S. Ogura, M. Inoue, Kagaku to Kyoiku (Chemical Education) 2013, 61, 86. (Japanese)
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.







