Caution Notice:
It has come to our notice that certain fraudulent individuals or entities are misusing our Company’s name and TCI’s registered trademarks by promoting and offering for sale regulated and hazardous chemical substances, including Potassium Cyanide, through online platforms like YouTube. We hereby categorically clarify that TCI has no association or connection whatsoever with the products being displayed or sold in these or similar videos. These products have been falsely represented as being associated with TCI, and the unauthorized use of our trademark and brand name is both illegal and misleading. TCI Chemicals is a responsible global organization committed to adhering to all applicable international and domestic regulatory frameworks. We do not manufacture, distribute, or engage in the sale of regulated chemical substances in India or in any other jurisdiction unless specifically authorized by the relevant laws and regulatory agencies. All our operations and offerings are governed by stringent safety, quality, and compliance protocols in accordance with applicable laws. Further, TCI Chemicals markets and sells its products exclusively through its official website and authorized distributors. We do not sell, endorse, or supply our products through any third-party platforms, unauthorized agents, or resellers. Any individual or organization purchasing products outside these official channels does so at their own risk, and TCI disclaims all responsibility and liability for any consequences arising therefrom. Members of the public, customers, and partners are strongly advised to exercise caution and conduct due diligence before engaging in any transactions involving products claiming association with TCI Chemicals. The official list of our authorized distributors is publicly available and may be accessed at: https://www.tcichemicals.com/IN/en/distributor/index. We are currently pursuing appropriate legal remedies against those misusing our brand, and we reserve all rights available to us under applicable intellectual property and criminal laws. If you become aware of any such fraudulent activity or require clarification, you may reach out to us at: Sales-IN@TCIchemicals.com. Your vigilance and cooperation are essential in safeguarding the public interest and protecting the integrity of the TCI brand.
Maximum quantity allowed is 999
Please select the quantity
Reduction of Alcohols
No.150(December 2012)
IPNBSH (1) is used in the reduction of alcohols to olefins, allenes and ketones under Mitsunobu reaction conditions (Table 1). Treatment of allylic alcohols with 1 forms olefins with the double-bond transposition. 1 is also used in the reduction of alkynyl alcohols and alkyl alcohols to give allenes and alkanes, respectively. Thermal stability of 1 is higher than that of 2-nitrobenzenesulfonyl hydrazide (NBSH), which has been conventionally used. The reduction using 1 can be performed around 0 °C to rt as compared to that using NBSH having to be carried out within –30 to –15 °C. 1 provided greater flexibility with respect to solvent choice, order of addition, and concentration of substrate and reagent. The reaction would proceed the forming of the diazene via the sulfonyl hydrazine, and subsequent loss of dinitrogen to give the reduction products.

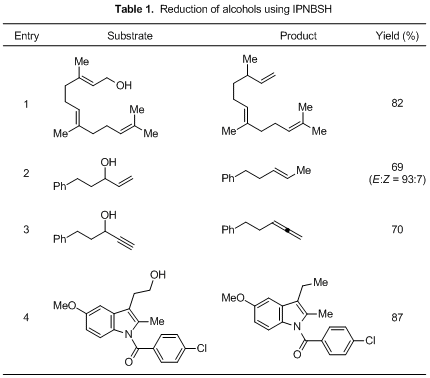
Typical Procedure: Synthesis of (E)-3,7,11-trimethyldodeca-1,6,10-triene (Table 1, Entry 1)
Diethyl azodicarboxylate (74 μL, 0.47 mL) is added dropwise to a mixture of IPNBSH (1, 122 mg, 0.474 mmol), trans,trans-fanesol (0.100 mL, 0.393 mmol) and triphenylphosphine (124 mg, 0.473 mmol) in anhydrous THF (9.0 mL) at 0 °C under an argon atmosphere. After 5 min, the reaction mixture is allowed to warm to 23 °C. After 20 min, a mixture of trifluoroethanol and water (1:1, 4.5 mL) is added to the reaction mixture. After 3 h, the reaction mixture is suspended between diethyl ether (25 mL) and water (25 mL), and the aqueous layer is extracted with diethyl ether. The combined organic layers are dried over anhydrous sodium sulfate, filtered, and concentrated. The residue is purified by flash column chromatography on silica gel (eluent: pentane) to give the desired triene (71 mg, Y. 87 %).
Diethyl azodicarboxylate (74 μL, 0.47 mL) is added dropwise to a mixture of IPNBSH (1, 122 mg, 0.474 mmol), trans,trans-fanesol (0.100 mL, 0.393 mmol) and triphenylphosphine (124 mg, 0.473 mmol) in anhydrous THF (9.0 mL) at 0 °C under an argon atmosphere. After 5 min, the reaction mixture is allowed to warm to 23 °C. After 20 min, a mixture of trifluoroethanol and water (1:1, 4.5 mL) is added to the reaction mixture. After 3 h, the reaction mixture is suspended between diethyl ether (25 mL) and water (25 mL), and the aqueous layer is extracted with diethyl ether. The combined organic layers are dried over anhydrous sodium sulfate, filtered, and concentrated. The residue is purified by flash column chromatography on silica gel (eluent: pentane) to give the desired triene (71 mg, Y. 87 %).
References
- N-Isopropylidene-N'-2-nitrobenzenesulfonyl hydrazine, a reagent for reduction of alcohols via the corresponding monoalkyl diazenes
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

