Caution Notice:
It has come to our notice that certain fraudulent individuals or entities are misusing our Company’s name and TCI’s registered trademarks by promoting and offering for sale regulated and hazardous chemical substances, including Potassium Cyanide, through online platforms like YouTube. We hereby categorically clarify that TCI has no association or connection whatsoever with the products being displayed or sold in these or similar videos. These products have been falsely represented as being associated with TCI, and the unauthorized use of our trademark and brand name is both illegal and misleading. TCI Chemicals is a responsible global organization committed to adhering to all applicable international and domestic regulatory frameworks. We do not manufacture, distribute, or engage in the sale of regulated chemical substances in India or in any other jurisdiction unless specifically authorized by the relevant laws and regulatory agencies. All our operations and offerings are governed by stringent safety, quality, and compliance protocols in accordance with applicable laws. Further, TCI Chemicals markets and sells its products exclusively through its official website and authorized distributors. We do not sell, endorse, or supply our products through any third-party platforms, unauthorized agents, or resellers. Any individual or organization purchasing products outside these official channels does so at their own risk, and TCI disclaims all responsibility and liability for any consequences arising therefrom. Members of the public, customers, and partners are strongly advised to exercise caution and conduct due diligence before engaging in any transactions involving products claiming association with TCI Chemicals. The official list of our authorized distributors is publicly available and may be accessed at: https://www.tcichemicals.com/IN/en/distributor/index. We are currently pursuing appropriate legal remedies against those misusing our brand, and we reserve all rights available to us under applicable intellectual property and criminal laws. If you become aware of any such fraudulent activity or require clarification, you may reach out to us at: Sales-IN@TCIchemicals.com. Your vigilance and cooperation are essential in safeguarding the public interest and protecting the integrity of the TCI brand.
Maximum quantity allowed is 999
Please select the quantity
Super Brønsted Acid
No.123(July 2005)

Products 1 and 2 are a super Brønsted acid and a polymer-supported super Brønsted acid, which are developed by Ishihara, Yamamoto and co-workers. These products have two trifluoromethanesulfonyl groups which are strong electron-withdrawing groups and one perfluorophenyl group on the methine carbon. The acidity of the proton on the methine carbon is remarkably high, and both products are utilized as excellent acid catalyst in various reactions.1a) For example, 1 has a pKa = 1.5 (in CD3CO2D) and it has been reported to be useful for the acylation of (-)-menthol.

2 is effectively swollen by both polar and nonpolar organic solvents, and it possesses high catalytic activity. It is used as a solid acid catalyst which can be recovered and reused. For example, in the acetalization of benzylacetone, it is quantitatively recovered after the reaction by simple filtration and it can be reused more than 10 times without loss of activity. The turnover number (TON) is greater than 10,000.1a)

Another application for 2 that has been reported is its use in a packed reaction column.1b) The reaction column is a rather simple system that consists of a disposable syringe packed with a mixture of 2 and Celite. Since the catalyst activity of 2 is quite high, it is possible to perform various acid-promoted reactions in high yield and in a single pass requiring just a short reaction time. For example, the Mukaiyama aldol reaction can be successfully performed by passing a mixed solution of benzaldehyde and the silylenol ether of acetophenone into the reaction column just once. The resulting silyl ether of the aldol product is again put through the reaction column as an aqueous THF solution, and hydrolysis progresses smoothly to give the desired aldol product in 96% yield over the 2-steps of procedures. Furthermore, the conversions into acetals and esters also progress readily, thereby making it a very useful reagent for functional group conversions of samples prior to NMR, HPLC and GC analyses. This reaction column can also be coupled to a pump to establish a continuous-flow reaction system.


The lithio product of 1 is known to undergo nucleophilic substitution at the para-position. Thus it is possible to create further designs. For example, 1H,1H-perfluorotetradecyloxy group was introduced at the para-position to obtain a product with a high fluorous property, which can be used as a fluorous super Brønsted acid catalyst 1a.1c) During the acetalization of benzaldehyde, 1a dissolves in cyclohexane when it is heated to reflux, and function as a catalyst. After the reaction, 1a precipitates upon cooling to room temperature, allowing it to be recovered and reused. As described herein, when 1a is utilized as homogeneous catalyst, its activity is higher than the solid catalyst 2; furthermore it can also be recovered as a solid catalyst, and there are advantages that it can be recovered and reused the catalyst without fluorous solvens.



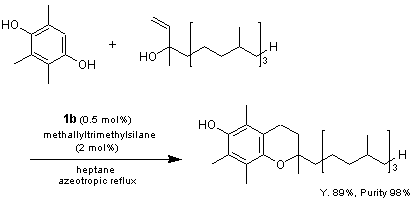
The trimethylsilyl pentafluorophenylbis(trifluoromethanesulfonyl)methide [(C6F5CTf2]SiMe3] 1b prepared from 1 and allyltrimethylsilane is utilized as a super Lewis acid catalyst in the regioselective condensation of trimethylhydroquinone with isophythol.1d) When the acidity of 1 is compared with TfOH and Tf2NH, the order of Brønsted acidity is TfOH > Tf2NH > 1, however, when the Lewis acidity of the trimethylated forms of these compounds was compared, the order of Lewis acidity becomes 1b > Tf2NSiMe3 > TfOSiMe3. It is anticipated that the conjugate base of 1 (C6F5C-Tf2) will find utility as a bulky counter anions.
References
- 1)Pentafluorophenylbis(triflyl)methane as a strong bronsted acid catalyst
- a)K. Ishihara, A. Hasegawa, H. Yamamoto, Angew. Chem. Int. Ed. 2001, 40, 4077.

- b)K. Ishihara, A. Hasegawa, H. Yamamoto, Synlett 2002, 1296.

- c)K. Ishihara, A. Hasegawa, H. Yamamoto, Synlett 2002, 1299.

- d)A. Hasegawa, K. Ishihara, H. Yamamoto, Angew. Chem. Int. Ed. 2003, 42, 5731.

- e)K. Ishihara, H. Yamamoto, TCI MAIL 2002, 115, 2.
- a)K. Ishihara, A. Hasegawa, H. Yamamoto, Angew. Chem. Int. Ed. 2001, 40, 4077.
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

