Caution Notice:
It has come to our notice that certain fraudulent individuals or entities are misusing our Company’s name and TCI’s registered trademarks by promoting and offering for sale regulated and hazardous chemical substances, including Potassium Cyanide, through online platforms like YouTube. We hereby categorically clarify that TCI has no association or connection whatsoever with the products being displayed or sold in these or similar videos. These products have been falsely represented as being associated with TCI, and the unauthorized use of our trademark and brand name is both illegal and misleading. TCI Chemicals is a responsible global organization committed to adhering to all applicable international and domestic regulatory frameworks. We do not manufacture, distribute, or engage in the sale of regulated chemical substances in India or in any other jurisdiction unless specifically authorized by the relevant laws and regulatory agencies. All our operations and offerings are governed by stringent safety, quality, and compliance protocols in accordance with applicable laws. Further, TCI Chemicals markets and sells its products exclusively through its official website and authorized distributors. We do not sell, endorse, or supply our products through any third-party platforms, unauthorized agents, or resellers. Any individual or organization purchasing products outside these official channels does so at their own risk, and TCI disclaims all responsibility and liability for any consequences arising therefrom. Members of the public, customers, and partners are strongly advised to exercise caution and conduct due diligence before engaging in any transactions involving products claiming association with TCI Chemicals. The official list of our authorized distributors is publicly available and may be accessed at: https://www.tcichemicals.com/IN/en/distributor/index. We are currently pursuing appropriate legal remedies against those misusing our brand, and we reserve all rights available to us under applicable intellectual property and criminal laws. If you become aware of any such fraudulent activity or require clarification, you may reach out to us at: Sales-IN@TCIchemicals.com. Your vigilance and cooperation are essential in safeguarding the public interest and protecting the integrity of the TCI brand.
Maximum quantity allowed is 999
Covalent organic frameworks (COFs) are crystalline organic frameworks consisting of a network structure made of covalent bonds.1,2) COFs are classified as porous crystalline materials similar to metal-organic frameworks (MOFs)/porous coordination polymers (PCPs) and zeolites. They include 2D COFs, which are constructed by stacking layers of 2D covalently bonded sheets, and 3D COFs, which are constructed by 3D connected frameworks. COFs are expected to be used as molecular storage or separation materials, catalysts, electronic materials, energy storage materials, battery materials, and drug delivery materials, due to their porosity, crystallinity, and structural diversity.
COFs are designed and synthesized by combining monomers, also called linkers, according to intended topology. TCI has more than 90 linkers in stock, and we are constantly adding new items to our catalog. Common linkers are shown below by functional groups.
Aldehyde Linkers
A type of COFs based on imine linkage, synthesized by condensation of aldehydes and amines, was first reported in 2009,3) and imine-based COFs have become the most widely reported COFs. One of the advantage of imine based COFs is their higher chemical stability compared to boroxines and boronate esters. In addition, a number of researchers have reported post-synthetic modification or functionalization of imine based COFs, e.g., COFs for CO2 capture were synthesized by post-synthetic modification and functionalization of imine-based structures.4) In 2012, β-ketoenamine-type COFs synthesized by using 2,4,6-triformylphloroglucinol (TPG, TFP) as an aldehyde linker were reported,5) and have recently attracted much attention because of their stability towards acids and bases.
Products
- T0010
- Terephthalaldehyde (= PDA)
- B2854
- 4,4'-Biphenyldicarboxaldehyde
- B6576
- [2,2'-Bipyridine]-5,5'-dicarbaldehyde
- D6103
- 2,3-Dihydroxyterephthalaldehyde
- D5510
- 2,5-Dihydroxyterephthalaldehyde
- D6056
- 2,5-Dimethoxyterephthalaldehyde
- T4088
- 2,3,5,6-Tetrafluoroterephthalaldehyde
- T3688
- 2,4,6-Triformylphloroglucinol (= TFP, TPG)
- D6046
- 2,4,6-Triformylresorcinol
- F1252
- 1,3,5-Tris(4-formylphenyl)benzene
- T4078
- 4,4',4''-(1,3,5-Triazine-2,4,6-triyl)tribenzaldehyde
- T4077
- 2,4,6-Tris(4-formylphenoxy)-1,3,5-triazine
Amine Linkers
Amine linkers are used to synthesize imine-linked COFs by condensation of aldehydes and amines as described in the aldehyde linkers section, as well as imide-linked COFs mentioned in the carboxylic anhydride linkers section below, and squaraine-linked COFs.6)
Products
- P0170
- 1,4-Diaminobenzene
- D2893
- [2,2'-Bipyridine]-5,5'-diamine
- D3180
- 2,6-Diaminoanthraquinone
- B6846
- 4,4'-(Benzo[c][1,2,5]thiadiazole-4,7-diyl)dianiline
- T2332
- Tris(4-aminophenyl)amine (= TAPA)
- T2728
- 1,3,5-Tris(4-aminophenyl)benzene (= TAPB)
- T3695
- 4,4',4''-(1,3,5-Triazine-2,4,6-triyl)trianiline
- T4341
- 4',4''',4'''''-(1,3,5-Triazine-2,4,6-triyl)tris[([1,1'-biphenyl]-4-amine)]
- M3538
- 2,5,8-Triamino-1,3,4,6,7,9,9b-heptaazaphenalene
- T2947
- Tetrakis(4-aminophenyl)methane
- T4075
- Tetrakis(4-aminophenyl)ethylene
- T1494
- 5,10,15,20-Tetrakis(4-aminophenyl)porphyrin
Carboxylic Anhydride Linkers
Imide-linked COFs obtained by condensation of carboxylic anhydrides and amines have also been reported7) and are expected to be applied to battery materials8) and CO2 capture materials.9)
Products
- B0040
- Pyromellitic Dianhydride
- P2103
- Pyromellitic Dianhydride (purified by sublimation)
- N1128
- 2,3,6,7-Naphthalenetetracarboxylic 2,3:6,7-Dianhydride
- N0369
- Naphthalene-1,4,5,8-tetracarboxylic Dianhydride
- N0755
- Naphthalene-1,4,5,8-tetracarboxylic Dianhydride (purified by sublimation)
- N1247
- 1,2,5,6-Naphthalenetetracarboxylic Dianhydride
- P0972
- 3,4,9,10-Perylenetetracarboxylic Dianhydride
- P2102
- 3,4,9,10-Perylenetetracarboxylic Dianhydride (purified by sublimation)
- M3617
- Mellitic Trianhydride
Boronic Acid Linkers
The self-condensation of boronic acids to produce boroxines and the condensation of boronic acids and catechols to produce boronic esters are the first synthetic strategies to synthesize COFs.10)
Products
Other Linkers
COFs can also be constructed with linkers beyond imines, imides, and boroxines. COFs prepared using linkers other than amines, aldehydes, carboxylic anhydrides, and boronic acids could include those utilizing hydrazones, azines, C=C double bonds, etc.
For example:
- Hydrazone-type COFs synthesized with hydrazides and aldehydes, are known for their structural flexibility and significant potential for post-synthetic modifications.11,12,13)
- Ionic COFs synthesized using 1,2,3-triaminoguanidinium chloride, where the triaminoguanidinium cation reacts with an aromatic aldehyde to form the covalent linkages within the framework.14)
- β-ketoenamine-type COFs can also be derived by synthesizing precursors with urea linkage. After the precursor with highly-reversible urea linkages is formed, the "reconstruction" process transforms it into the final β-ketoenamine COF. Researchers can achieve a significantly higher crystallinity and surface area in the final β-ketoenamine COF by using this "precursor approach" with urea linkages.15)
Products
- H0907
- 2,3,6,7,10,11-Hexahydroxytriphenylene (= HHTP)
- B6577
- 1,1'-([1,1'-Biphenyl]-4,4'-diyl)diurea
- T0758
- Terephthalic Dihydrazide
- H0172
- Hydrazine Monohydrate
- H0697
- Hydrazine Anhydrous
- T4080
- 1,2,3-Triaminoguanidinium Chloride
- D1399
- Squaric Acid
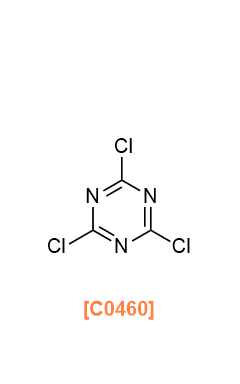
- C0460
- 2,4,6-Trichloro-1,3,5-triazine
- T4145
- 2,5,8-Trichloro-1,3,4,6,7,9,9b-heptaazaphenalene
- X0061
- 1,4-Phenylenediacetonitrile
- T0016
- Terephthalonitrile
Related Products
The following is a list of common reagents that are used as modulators to increase the crystallinity of the resulting COFs and as catalysts for the synthesis of COFs.
Modulators
Catalysts
Examples of COFs Synthesis
Synthesis of COF-300 3)

Synthesis of TpPa-1 5)

Synthesis of COF-1 10)

Synthesis of Bth-Dma COF 13)

Synthesis of RC-COF-3 15)

Product Brochures
Covalent Organic Framework (COF) Linkers (PDF file)
Aldehyde Linkers for β-Ketoenamine Linked Covalent Organic Frameworks (COFs) (PDF file)
Raw Material for Polyimides and COFs Mellitic Trianhydride (PDF file)
2,2'-Bipyridine Linkers for the Synthesis of COFs (PDF file)
Diurea Linkers for the Synthesis of Reconstructed COFs (PDF file)
Hydrazide Linkers for Covalent Organic Flameworks (COFs) (PDF file)
References
- 1) Covalent Organic Frameworks: Organic Chemistry Extended into Two and Three Dimensions
- 2) Covalent Organic Frameworks: Structures, Synthesis, and Applications
- 3) A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework
- 4) Covalent Organic Frameworks for Carbon Dioxide Capture from Air
- 5) Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route
- 6) A Squaraine-Linked Mesoporous Covalent Organic Framework
- 7) Designed synthesis of large-pore crystalline polyimide covalent organic frameworks
- 8) Covalent Organic Framework with Highly Accessible Carbonyls and π-Cation Effect for Advanced Potassium-Ion Batteries
- 9) Synthesis, characterization, and CO2 uptake of mellitic triimide-based covalent organic frameworks
- 10) Porous, Crystalline, Covalent Organic Frameworks
- 11) Crystalline Covalent Organic Frameworks with Hydrazone Linkages
- 12) Hydrazone-Linked Covalent Organic Frameworks
- 13) Stable Hydrazone-Linked Covalent Organic Frameworks Containing O,N,O'-Chelating Sites for Fe(III) Detection in Water
- 14) Cationic Covalent Organic Framework Nanosheets for Fast Li-Ion Conduction
- 15) Reconstructed covalent organic frameworks





![[2,2'-Bipyridine]-5,5'-dicarbaldehyde](https://www.tcichemicals.com/assets/cms-images/covalent_organic_frameworks_COFs_linkers_B6576.png)










![[2,2'-Bipyridine]-5,5'-diamine](https://www.tcichemicals.com/assets/cms-images/covalent_organic_frameworks_COFs_linkers_D2893.png)

![4,4'-(Benzo[c][1,2,5]thiadiazole-4,7-diyl)dianiline](https://www.tcichemicals.com/assets/cms-images/covalent_organic_frameworks_COFs_linkers_B6846.png)



![4',4''',4'''''-(1,3,5-Triazine-2,4,6-triyl)tris[([1,1'-biphenyl]-4-amine)]](https://www.tcichemicals.com/assets/cms-images/covalent_organic_frameworks_COFs_linkers_T4341.png)















![1,1'-([1,1'-Biphenyl]-4,4'-diyl)diurea](https://www.tcichemicals.com/assets/cms-images/covalent_organic_frameworks_COFs_linkers_B6577.png)
![1,1',1''-[(1,3,5-Triazine-2,4,6-triyl)tris(benzene-4,1-diyl)]triurea](https://www.tcichemicals.com/assets/cms-images/covalent_organic_frameworks_COFs_linkers_T4192.png)