It has come to our notice that certain fraudulent individuals or entities are misusing our Company’s name and TCI’s registered trademarks by promoting and offering regulated and hazardous chemical substances through online platforms like YouTube. We hereby categorically clarify that TCI has no association or connection whatsoever with the products being displayed or sold in the videos. These products have been falsely represented as being associated with TCI, and the unauthorized use of our trademark and brand name is both illegal and misleading. TCI Chemicals markets and sells its products exclusively through its official website and authorized distributors. If you become aware of any such fraudulent activity or require clarification, you may reach out to us at: Sales-IN@TCIchemicals.com. Click Here to View the Caution Notice.
Product Document Searching Made Easy by 2D Code! | [Product Highlights] Endogenous Biotin-Blocking Reagent...Maximum quantity allowed is 999
Please select the quantity
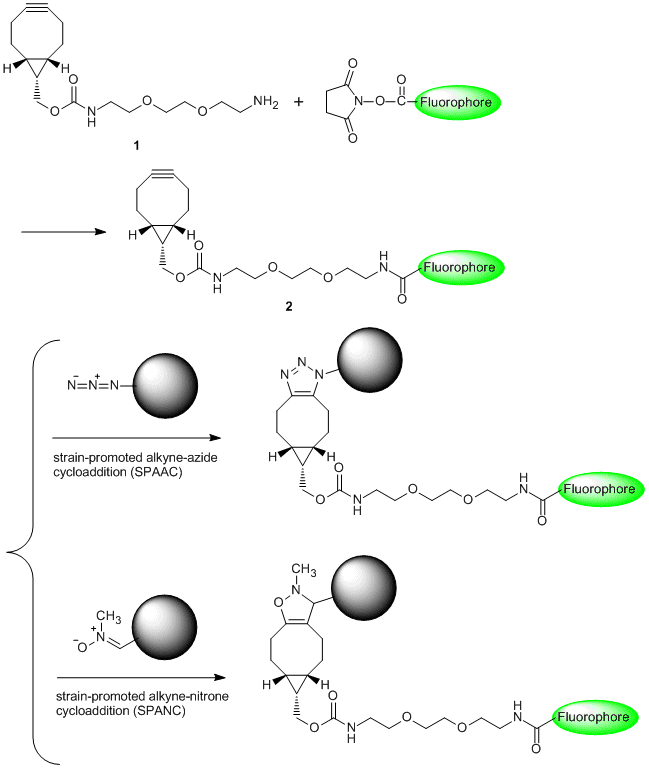
A Bioconjugatable Linker having a Copper-free "Click" Reaction to Azides
Huisgen [3+2] cycloaddition of azides with terminal alkynes is representative of "Click Chemistry" described by K. B. Sharpless in 2001. The reaction which affords products in high yield has been used for imaging labeling and tracking labeling of biomolecules. However, the reaction is not suitable for labeling of living systems because it needs a highly-concentrated copper(I) species.1)
BCN-amine (1) is a linker having a strained structure with cyclooctyne, and it is used for the copper-free click reaction to azides. For example, 1 bonded to a fluorophore (2) has resulted in labeling of an azidohomoalanine-containing virus capsid protein without copper(I) species.2) In addition, 1 can be applied to not only strain-promoted alkyne-azide cycloaddition (SPAAC)3) but also strain-promoted alkyne-nitrone cycloaddition (SPANC).4)
BCN-amine (1) is a linker having a strained structure with cyclooctyne, and it is used for the copper-free click reaction to azides. For example, 1 bonded to a fluorophore (2) has resulted in labeling of an azidohomoalanine-containing virus capsid protein without copper(I) species.2) In addition, 1 can be applied to not only strain-promoted alkyne-azide cycloaddition (SPAAC)3) but also strain-promoted alkyne-nitrone cycloaddition (SPANC).4)
References
- Copper-free azide–alkyne cycloadditions: new insights and perspectives
- Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells
- A strain-promoted [3 + 2] azide–alkyne cycloaddition for covalent modification of biomolecules in living systems
- Protein modification by strain-promoted alkyne–nitrone cycloaddition

